Abstract
Many extant species are at risk to go extinct. This impending loss of species is likely to cause changes in future ecosystem functions. Ecological components of diversity, such as dietary or habitat specializations, can be used to estimate the impact of extinctions on ecosystem functions. As an approach to estimate the impact of future extinctions, we tested interdependency between ecological and taxonomic change based on current predictions of extinction rates in primates. We analyzed the ecological characteristics of extant primate faunas having species in various categories of endangerment of extinction and forecasted the future primate faunas as if they were paleontological faunas. Predicting future faunas combines the wealth of ecological information on living primates with large, fossil record-like changes in diversity. Predicted extinction patterns of living primates in Africa, Asia, Madagascar, and South America show that changes in ecology differ among the regions in ways that are not reducible to taxonomic measures. The ecological effects of primate extinctions are initially least severe in South America and larger in Asia and Africa. Disproportionately larger ecological changes are projected for Madagascar. The use of taxonomy as a proxy for ecology can mislead when estimating competence of future primate ecosystems.
Diversity of future ecosystems can be predicted to decline because present-day extinction rates of organisms may rival those in the fossil record and many living species are at risk to go extinct in the near future (1, 2). In extant ecosystems, ecological attributes of species can influence ecosystem processes more than number of species (species richness) per se (3–5). For example, loss of all of the species that perform a specific ecological function, such as seed dispersal, may limit long-term ecosystem competence. Therefore, full appraisal of the impact of loss in biological diversity requires the inclusion of ecological components of diversity.
To simulate large time scale (i.e., paleontological or macroevolutionary) diversity losses using living faunas, in this paper we introduce an approach. We analyzed the ecological characteristics of extant faunas having species in various categories of endangerment of extinction and forecasted ecological integrity of faunas in the future, assuming extinctions proceed according to current rankings of endangerment. Predicting future faunas allows us to circumvent the problem that living species represent only one geological instant in the history of earth and fossil record-like changes in diversity cannot be directly observed. On the other hand, the use of extant fauna avoids the problem that fossils provide limited information on the ecological components of diversity, such as dietary or habitat specializations. These factors can be known from extinct species only indirectly via morphology (6–10).
Of mammals, the order Primates has the highest proportion of endangered species (11, 12), mainly because of human-caused habitat destruction and hunting (11–16). Ecology of primate species is extensively studied and primates play a key role in many ecosystem processes (17–20). First, we analyzed ecological richness (number of ecological types such as dietary specializations) and ecological composition of extant primate faunas in four geographical regions. Ecological composition is conceptually different from richness and characterizes the resource use (e.g., diet and habitat) of faunas. This allows measuring of ecological dissimilarity and range of adaptations. Then, using endangerment categories, we made projections of the integrity of extant primate faunas in the future and tested, using random sampling, interdependency between ecological and taxonomic change. This analysis can also be used to evaluate conservation actions on biodiversity that use taxonomy as a proxy for ecology.
METHODS
By assuming no future success in conservation actions, we generated a crude approximation of impending primate communities by ranking extant primates into three consecutive faunas based on their risks of extinction. The faunas are as follows: the present fauna (P) that incorporates all living primates, the first after the present fauna (AP I) that lacks all of the endangered species, and the second after the present fauna (AP II) that has only the presently nonthreatened species left. We used largely World Conservation Union (IUCN) and also United States Endangered Species Act (USESA) categories (21–24) to classify primates into different extinction sequence groups. Critically endangered (IUCN) and endangered (IUCN, USESA) primates were classified to go extinct first (AP I). Vulnerable (IUCN) and threatened (USESA) primates were classified to go extinct next (AP II). Abundant, nonthreatened, and lower risk categories were classified not to go extinct. Only three consecutive faunas were formed because population viability assessments of extinction risks are imprecise in estimating the actual time to extinction (25, 26). While we used these divisions to project the primate faunas as distinct faunal assemblages, these extinctions could appear to be instantaneous (with no time for speciation) in the paleontological time scale (2, 27).
To tabulate ecological richness, we used ecological diversity measures that cut across taxonomic boundaries. We characterized primary resource use of living African, Asian, Malagasy, and South American (including Central American) primates by tabulating their diet, activity pattern, and habitat as discrete specialization types and also their body size (according to the method in refs. 9 and 28). As a robust measure of specialization, dietary and habitat resources were ranked in order of importance. The tabulated primary diets (seven diets) were insects, meat, flowers/nectar, gum, fruits, seeds, and fibrous vegetation. To avoid incidental diet items, only the first five ranked primary diets were used. The tabulated primary habitats (five habitats) were tropical rain forests, deciduous forests, swamps or flooded forests, scrub or scrub forests, and open habitat (savanna). Similar ranked tabulation was used for activity pattern (diurnal, nocturnal) and spatial location (arboreal, terrestrial). Because our categories refer only to primate resource use apart from morphology and locomotion, it is readily applicable to other groups (for example, birds and bats would have “volant” spatial location). The mean body weights of species were used. The ecological data were largely derived from the compilations in ref. 24 and the taxonomic categories are according to ref. 29.
As a morphological richness measure that can be related to diet (refs. 9 and 30 and references therein), we tabulated primate molar tooth crown types (9). We used several collections (mainly American Museum of Natural History, New York, NY, and Duke Primate Center, Durham, NC) to assign species to crown types. Of the four regions, Madagascar has experienced severe primate extinctions in the Holocene (11) and we combined taxonomic and crown type richness of the subfossil lemurs and living taxa as a before present fauna.
To test whether the decline in the number of morphological or ecological types per se can be considered to be random among species, we pulled 1,000 random specific-sized subsamples (the number of species at AP I and AP II) from the present continental faunas and determined the probability of obtaining the projected parameter from the rarefaction frequency distributions (two tailed). Because of missing data the sampling was done separately for each variable (total number of species for each present fauna were 51–67, Africa; 54–59, Asia; 31–32, Madagascar; 61–74, South America).
To tabulate ecological composition (measured as the degree and direction to which species differ ecologically), we reduced the dimensionality of the data by ordinating specialization types of 192 species using principal component analysis. Variables within each four ecological category (activity cycle, spatial location, diet, and habitat tabulations) were given values based on their rank order. For example, the first diet of species with three primary diets was assigned value “three,” the second “two,” and the third “one.” Because categories and species had different numbers of variables, each category was standardized by dividing it by the sum of values (the example diet values are 3/6 + 2/6 + 1/6 = 0.5 + 0.33 + 0.17 = 1). This tabulation puts relatively more weight on the high-ranked primary resource when the species are specialized (uses only few primary diets or habitats). Body size, an important component of an animal’s ecology, was included in these analyses (log transformed). Variances for each variable were made equal by standardization by standard deviations (z scores). Patterns were broadly similar without z score standardization or body size. Ordination was done from correlation matrix using SYN-TAX 5.02 (31). Since all species-species contrasts shared variables (in addition to body size) and most (94%) of species-species contrasts shared at least four variables, flexible shortest path adjustments of distant matrix (31) did not markedly change the original ordination used in the analysis.
To measure the changes in continental level ecological composition, we tabulated changes in ecological ranges and average positions (ecological centroids) of the continental primate faunas in the ecospace. Ecological ranges were tabulated as the sum of the ranges of the three first or all 17 factors (for discussion on methods, see ref. 32). The probability of obtaining projected parameters by random extinction was obtained from rarefaction frequency distribution (1,000 random specific-sized subsamples). Note that the centroid position does not change when the extinctions are random. Faunal disparities (as average euclidean distances) were calculated using all factors.
We compared ecological disparities of primate species to their corresponding phylogenetic distances to examine whether the degree to which species differ ecologically is closely associated with the degree of their independent evolutionary history. For a robust measure of phylogenetic distance, we tabulated, using 10 million-yr intervals, durations of independent evolutionary history of each primate species from a composite phylogeny (33).
RESULTS
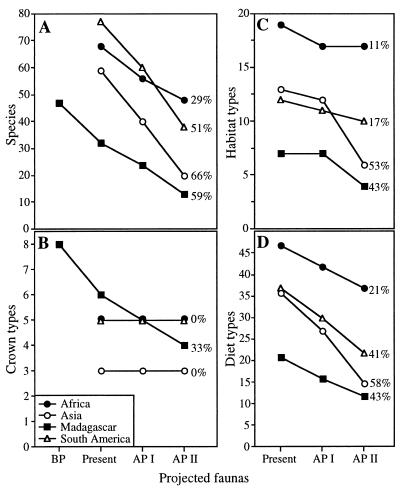
By the AP II, all four regions are projected to lose 29–66% of their extant primate species (Fig. 1A), with Asia and Madagascar experiencing the more severe extinctions. Tabulations of molar crown-type richness (Fig. 1B) show only a decline in Madagascar, but habitat- and diet-type richness (Fig. 1 C and D) show greater loss in Asia and Madagascar than in Africa and South America. The number of projected crown, diet, and habitat types were not significantly different from the rarefaction frequency distributions (crown types, P = 0.3–1.0; habitat types, P = 0.1–0.6; diet types, P = 0.08–0.6), and, thus, the decline in the species richness roughly approximates the decline in morphological and ecological richness (Fig. 1).
Figure 1.
Species (A), molar crown-type (B), habitat-type (C), and diet-type (D) richness trend projections for each continent. The Holocene subfossil primate richness of Madagascar is also reconstructed (A and B). The amounts of decline (percent) from present diversities are marked next to the slopes. BP, before present; AP I, after present I; AP II, after present II.
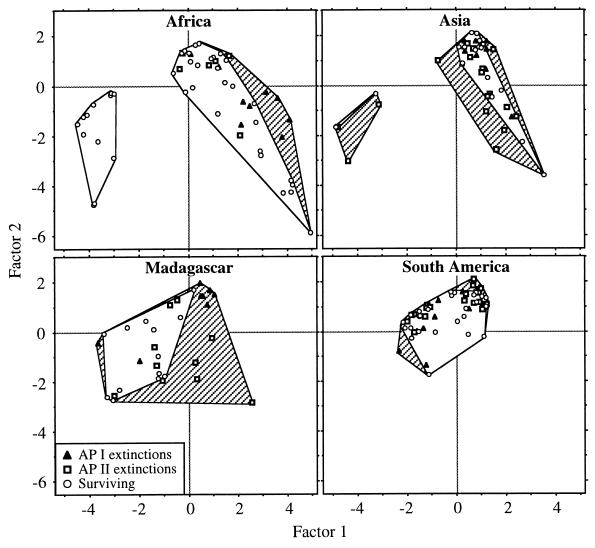
In Fig. 2, ecological composition of primate faunas, as depicted by principal component analysis, is shown for the first three factors. The first three factors account for 24.2%, 16.3%, and 12.6%, respectively, of the total variance. The first factor mainly corresponds to primate life history traits that are associated with body size, but the second factor is more indicative of the habitat type, and the third characterizes diet (Table 1).
Figure 2.
Principal component ordination of the first two factors showing the ecological distribution of primates. Hatched polygons approximate ecological ranges that are projected to be vacated after extant primate extinctions. Note the general resemblance between African and Asian primate specializations which have separate nocturnal (left) and diurnal (right) guild members. The lower parts of the diurnal specializations contain more terrestrial, open habitat primates (such as baboons in Africa) and the upper parts contains more arboreal, rain forest primates. Malagasy and South American primates have partly intermediate specializations. The most severe change in ecological range is projected to happen in Madagascar, while Africa has less severe, but ecologically specific extinctions. Loss in the ecological range of Asian primates is severe but only a little more severe than would be expected based on the decline in Asian primate species richness (Table 2). South American extinctions affect taxonomic more than ecological aspects of diversity.
Table 1.
Component correlations of each variable for the first three factors
| Variable | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| Diurnal | 0.715 | 0.418 | 0.318 |
| Nocturnal | −0.715 | −0.418 | −0.318 |
| Arboreal | −0.647 | 0.590 | −0.288 |
| Terrestrial | 0.646 | −0.590 | 0.288 |
| Rain forest | −0.254 | 0.638 | 0.326 |
| Deciduous | 0.032 | −0.510 | −0.569 |
| Wet | 0.113 | 0.300 | 0.302 |
| Scrub | 0.108 | −0.433 | −0.087 |
| Open | 0.409 | −0.630 | 0.166 |
| Fruit | 0.131 | 0.328 | 0.074 |
| Fibrous | 0.400 | 0.191 | −0.605 |
| Seeds | 0.506 | −0.055 | 0.152 |
| Flowers/nectar | 0.131 | 0.142 | −0.423 |
| Gum | −0.451 | −0.241 | 0.111 |
| Insects | −0.670 | −0.313 | 0.469 |
| Meat | −0.331 | −0.172 | 0.633 |
| Body size | 0.888 | 0.144 | −0.038 |
Despite the projected steep decline in primate taxonomic- and ecological-type richness (Fig. 1), the ranges of ecological specializations show only a moderate decline (0–22% for the first three factors, Table 2). Moreover, the ranges (including the total ranges of 17 factors) do not differ significantly from rarefaction estimates (Table 2). However, directional extinctions of primate specializations, measured with ecological centroids of primate faunas, show significant trends (Table 2). In Africa the value of the first factor centroid shows an apparent decrease (Table 2), as larger, more terrestrial (Mandrillus, Pan, and Gorilla), as also smaller folivorous and frugivorous primates go extinct (colobines and Cercocebus, Fig. 2). The data show that in Asia the extinctions are ecologically more disruptive; diurnal arboreal specializations (mainly Hylobates) decrease in number, as do more folivorous primates (mainly Pygathrix and Trachypithecus). In Madagascar, the decline in ecological range is strongly directional as broad groups of diurnal specializations disappear (Fig. 2 and Table 2). Moreover, the average ecological distance (disparity) among primate species declines and thus more ecologically different primates go extinct in Madagascar, whereas in other regions more ecologically similar primates go extinct. South American primates are projected to show very little change in ecological composition (Fig. 2 and Table 2) despite the halving of its taxonomic diversity (Fig. 1).
Table 2.
Predicted changes in the continental level ecological composition of recent primate extinctions
| Continent, projected fauna | Ecological range | Ecological centroid
|
Mean euclidean distance (SD) | Mean no. of habitats (SD) | ||
|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | ||||
| Africa, P | 23.17 | 0.56 | −0.77 | 0.56 | 6.23 (2.20) | 1.88 (0.84) |
| Africa, AP I | 23.17 (NS) | 0.19* | −0.81 (NS) | 0.51 (NS) | 6.22 (2.32) | 1.93 (0.81) |
| Africa, AP II | 23.17 (NS) | 0.09* | −1.00 (NS) | 0.62 (NS) | 6.45 (2.34) | 2.00 (0.83) |
| Asia, P | 18.48 | 0.42 | 0.30 | −0.12 | 4.79 (1.98) | 1.64 (0.80) |
| Asia, AP I | 18.48 (NS) | 0.27 (NS) | 0.04* | 0.04 (NS) | 5.13 (1.98) | 1.80 (0.85) |
| Asia, AP II | 17.22 (NS) | 0.25 (NS) | 0.32 (NS) | 0.22 (NS) | 4.99 (2.17) | 1.75 (0.85) |
| Madagascar, P | 15.64 | −0.92 | −0.44 | −1.83 | 5.42 (1.61) | 1.31 (0.59) |
| Madagascar, AP I | 14.93 (NS) | −1.18 (NS) | −0.92*** | −2.23*** | 5.34 (1.57) | 1.33 (0.64) |
| Madagascar, AP II | 12.41 (NS) | −1.78** | −0.84 (NS) | −2.29 (NS) | 4.91 (1.31) | 1.15 (0.38) |
| South America, P | 13.50 | −0.41 | 0.77 | 0.54 | 4.35 (1.32) | 1.76 (0.74) |
| South America, AP I | 13.35 (NS) | −0.40 (NS) | 0.80 (NS) | 0.53 (NS) | 4.37 (1.35) | 1.81 (0.76) |
| South America, AP II | 12.82 (NS) | −0.51 (NS) | 0.61 (NS) | 0.46 (NS) | 4.55 (1.44) | 1.86 (0.80) |
Ecological range was tabulated as the sum of the ranges of the three first factors and ecological centroid was the mean value of each factor. NS, P > 0.05; ∗, 0.05 ≥ P > 0.01; ∗∗, 0.01 ≥ P > 0.001; ∗∗∗, P ≤ 0.001 for obtaining the AP I or AP II parameters from frequency distributions of subsamples that were pulled randomly 1000 times from the present (P) faunas.
Habitat loss increases the risk of extinction (1) and may affect primates that are habitat specialists more than habitat generalists (34, 35). To estimate the degree of habitat specialization, we tabulated the average number of primary habitats used by each primate species. The average number of habitats per species is projected to increase in Africa, Asia, and South America (Table 2). Thus, primates that specialize in one primary habitat are more likely to go extinct [even if habitats are destroyed randomly (1)]. In contrast, in Madagascar, primates that specialize in many primary habitats are more likely to go extinct.
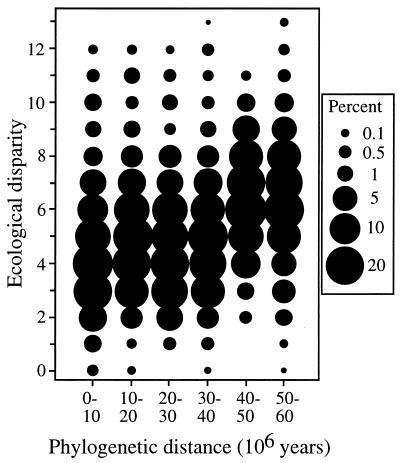
The degree of independent evolutionary history shows very little effect on ecological disparities until lineages that have been independent more that 40 million yr are reached (Fig. 3). This basically corresponds to the split between prosimians and higher primates, and thus even the origin of modern primate families is not visible in the ecological differences among taxa. This suggests that parallel and convergent evolution to ecologically similar life modes has been rampant among primates.
Figure 3.
Primate ecological disparity as a function of phylogenetic distance. The size of each dot represents the relative frequency of a particular ecological disparity value (plotted as rounded euclidean distance) for each phylogenetic distance category (as 10 million-yr intervals). Note the similar ecological disparities until more than 40 million-yr-old primate lineages and relatively high frequency of very disparate (disparity >10) primates among phylogenetically young lineages. The ecological disparity averages are for each time interval: 0–10 = 4.5, 10–20 = 5.0, 20–30 = 4.7, 30–40 = 5.0, 40–50 = 6.4, and 50–60 = 6.4.
DISCUSSION
Large changes in primate ecological composition resulting from extinctions require attention. Ecological composition, which measures the degree and direction of ecological differences among primates, depicts ecosystem organization. Species in an ecosystem can have ecologically comparable specializations, in which case the ecosystem organization can be considered simple or specializations can be disparate, in which case the species interactions are complex. None of the studied primate faunas have identical ecological composition and thus, from the point of ecosystem organization, the simple taxonomic or ecological richness measures are not necessarily comparable among the regions. The most similar composition is found between African and Asian primates. The distributions of African and Asian primate specializations (i.e., niches) in the ordinated “ecospace” resemble each other (Fig. 2) in that the small body-sized, nocturnal, and largely insectivorous specializations form one group, and the larger, diurnal specializations form a separate group [guild or functional groups (36, 37)]. Although the compositional similarity between Africa and Asia agrees with the linked evolutionary history of these two continents (38), the evolutionary history of Madagascar and South America are very distinct, but their primates have partly overlapping, intermediate specializations.
Furthermore, the degree of independent evolutionary history among primate taxa gives an inaccurate description of the degree of their ecological differences. For example, ecologically disparate primates are relatively as common among taxa that are phylogenetically close as among phylogenetically distant taxa (Fig. 3). Therefore, primate phylogeny has largely incidental correlation with ecological composition, and, as in the fossil record (9, 39), different measures depict different aspects of biodiversity.
The use of taxonomy as a proxy for ecology can be especially misleading when changes in primate ecological composition are large. In particular, disproportionately large alterations in ecosystem integrity can be predicted when changes in the ecological composition of primate faunas exceed changes predicted by the loss of species richness (Table 2). This can happen, for example, when specific specializations are lost. At least for plant ecosystem processes, ecological characteristics of individual species have been shown to rival the importance of species diversity (4, 5). Likewise, primate species can have critical ecological roles, in particular as plant pollinators and seed dispersers and can be used to estimate the overall human impact on ecosystems (17–20). This also stresses the importance of acquisition and inclusion of ecological data in successful conservation of biodiversity.
By far the largest changes in ecological composition are projected for Madagascar (Table 2). The Malagasy extinctions affect mostly diurnal specializations and are accompanied by the loss of primates with more generalized use of habitats. This could, for example, be the result of the final decimation of suitable habitats for Malagasy primates and mark the extinction of broad groups of diurnal specializations to folivory and frugivory (Fig. 2). Based on taxonomic- and crown-type diversity (Fig. 1), the ecological collapse in Madagascar may be a continuation of past extinctions (10) and Malagasy primates are “one step ahead” of the other regions.
Although African primates are predicted to be taxonomically least prone to go extinct, these extinctions are ecologically selective (Table 2 and Fig. 2). This is largely due to the loss of large bodied and terrestrial great apes. In contrast, Asian primate extinctions are predicted to be taxonomically the most severe but the changes in their ecological composition are only moderately more severe than predicted based on the loss of species (Table 2). This could be due to the loss of taxa on islands (e.g., Mentawai and Philippine islands) which may have ecologically resembling taxa left in other areas. Also, South American primates are predicted to have taxonomically severe extinctions but with very little change in their ecological composition. This suggests that only South American primate extinctions may qualify as ecologically random (32, 40) in the sense that, for example, a partial loss of the total habitat suitable for South American primates to live in results in the loss of taxa that have ecological equivalents elsewhere. Therefore, local ecosystems can experience severe changes in their ecological composition even though continental composition remains initially unchanged. Such threatened local ecosystems include the Atlantic forest of Brazil (41, 42). Additionally, South American primates appear to be ecologically the most uniform group (Fig. 2; see also ref. 43) and other mammalian taxa may substitute for the remaining primate specializations (44) and also experience ecologically selective extinctions.
In conclusion, we have shown that the decline in continental level taxonomic diversity of primates may have substantially different effects on primate ecological composition. In South America, a loss of more than half of their primate taxa may initially result in relatively small ecological changes. The ecological effects of primate extinctions are relatively larger in Asia and Africa. In Madagascar, primate extinctions may be ecologically the most severe. Malagasy primates make up 44% of the nonvolant terrestrial mammalian taxa (8–12% in other regions, only taxa found within the ranges of primates tabulated). This can substantially increase the directional effects (45) of Malagasy primate extinctions on ecosystem processes and also make lemurs more prone to go extinct. It is noteworthy that our tabulations are based on predicted species extinctions. Because population level extinction rates are higher than species extinction rates (46), the projected shrinking of the ecological composition depicts the “last refuge scenario.”
Acknowledgments
We thank F. van Berkum, M. Fortelius, I. Hanski, J. Hunter, C. Janson, J. Laakkonen, J. Lindström, P. Nieminen, and J. Oates for comments or advise on various versions of this paper. D. LaSilva assisted with the data entry.
ABBREVIATIONS
- AP I
first after the present fauna
- AP II
second after the present fauna
- IUCN
World Conservation Union
- USESA
United States Endangered Species Act
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Pimm S L, Russell G J, Gittleman J L, Brooks T M. Science. 1995;269:347–350. doi: 10.1126/science.269.5222.347. [DOI] [PubMed] [Google Scholar]
- 2.Sepkoski J J., Jr J Paleont. 1997;71:533–539. doi: 10.1017/s0022336000040026. [DOI] [PubMed] [Google Scholar]
- 3.Myers N. Proc Natl Acad Sci USA. 1996;93:2764–2769. doi: 10.1073/pnas.93.7.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper D U, Vitousek P M. Science. 1997;277:1302–1305. [Google Scholar]
- 5.Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E. Science. 1997;277:1300–1302. [Google Scholar]
- 6.Janis C M. Annu Rev Ecol Syst. 1993;24:467–500. [Google Scholar]
- 7.Van Valkenburgh B. In: Ecological Morphology. Wainwright P T, Reilly S M, editors. Chicago: Univ. of Chicago Press; 1994. pp. 140–168. [Google Scholar]
- 8.Fortelius M, Werdelin L, Andrews P, Bernor R L, Gentry A, Humphrey L, Mittmann H-W, Viranta S. In: The Evolution of Western Eurasian Neogene Mammal Faunas. Bernor R L, Fahlbusch V, Mittmann H-W, editors. New York: Columbia Univ. Press; 1996. pp. 414–448. [Google Scholar]
- 9.Jernvall J, Hunter J P, Fortelius M. Science. 1996;274:1489–1492. doi: 10.1126/science.274.5292.1489. [DOI] [PubMed] [Google Scholar]
- 10.Godfrey L R, Jungers W L, Reed K E, Simons E L, Chatrath P S. In: Natural Change and Human Impact in Madagascar. Goodman S M, Patterson B R, editors. Washington: Smithsonian Institution Press; 1997. pp. 218–256. [Google Scholar]
- 11.Mittermeier R A, Tattersall I, Konstant W R, Meyers D M, Mast R B. Lemurs of Madagascar. Washington, DC: Conservation International; 1994. [Google Scholar]
- 12.Ceballos G, Brown J H. Conserv Biol. 1995;9:559–568. [Google Scholar]
- 13.Johns A D, Skorupa J P. Int J Primatol. 1987;8:157–191. [Google Scholar]
- 14.Oates J F. Aust J Ecol. 1996;21:1–9. [Google Scholar]
- 15.Struhsaker T T. Ecology of an African Rain Forest. Gainesville: University Press of Florida; 1997. [Google Scholar]
- 16.Terborgh J W, Van Schaik C P. In: Last Stand. Kramer R C, Van Schaik C P, Johnson J, editors. New York: Oxford Univ. Press; 1997. pp. 15–35. [Google Scholar]
- 17.Janson C H. Science. 1983;219:187–189. doi: 10.1126/science.219.4581.187. [DOI] [PubMed] [Google Scholar]
- 18.Bourlieré F. Int J Primatol. 1985;6:1–26. [Google Scholar]
- 19.Chapman C A. Evol Anthropol. 1995;4:74–82. [Google Scholar]
- 20.Dew, J. L. & Wright, P. C. (1998) Biotropica 30, in press.
- 21.Mace G M. In: Extinction Rates. Lawton J H, May R M, editors. Oxford: Oxford Univ. Press; 1995. pp. 197–213. [Google Scholar]
- 22.Baillie J, Groombridge B, Gärdenforf U. IUCN Red List of Threatened Animals. Gland: IUCN; 1996. [Google Scholar]
- 23.Oates J F. Status Survey and Conservation Action Plan African Primates. Gland: IUCN; 1996. [Google Scholar]
- 24.Rowe N. The Pictorial Guide to the Living Primates. New York: Pogonias Press; 1996. [Google Scholar]
- 25.Taylor B L. Conserv Biol. 1995;9:551–558. [Google Scholar]
- 26.Harcourt A H. Conserv Biol. 1995;9:134–142. [Google Scholar]
- 27.Hunter J P. J Paleont. 1994;68:1158–1159. [Google Scholar]
- 28.Hunter J P, Jernvall J. Proc Natl Acad Sci USA. 1995;92:10718–10722. doi: 10.1073/pnas.92.23.10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson D E, Reeder D M. Mammalian Species of the World: A Taxonomic and Geographic Reference. Washington, DC: Smithsonian Institution Press; 1993. [Google Scholar]
- 30.Janis C M. Zoology. 1997;100:203–220. [Google Scholar]
- 31.Podani J. Multivariate Data Analysis in Ecology and Systematics. Hague, The Netherlands: SPB Academic Publishing; 1994. [Google Scholar]
- 32.Foote M. Paleobiology. 1992;18:1–16. [Google Scholar]
- 33.Purvis A. Philos Trans R Soc London B. 1995;348:405–421. doi: 10.1098/rstb.1995.0078. [DOI] [PubMed] [Google Scholar]
- 34.Emmons L H, Gautier-Hion A, Dubost G. J Zool. 1983;199:209–222. [Google Scholar]
- 35.Peres C A. J Trop Ecol. 1993;9:259–276. [Google Scholar]
- 36.Simberloff D, Dayan T. Annu Rev Ecol Syst. 1991;22:115–143. [Google Scholar]
- 37.Martinez N D. In: Biodiversity: A Biology of Numbers and Differences. Gaston K J, editor. Cambridge: Blackwell Science; 1996. pp. 114–148. [Google Scholar]
- 38.Martin R D. Primate Origins and Evolution: A Phylogenetic Analysis. Princeton: Princeton Univ. Press; 1990. [Google Scholar]
- 39.Hunter J P. In: Encyclopedia of Paleontology. Singer R, editor. Fitzroy Dearborn Publishers; 1998. , in press. [Google Scholar]
- 40.Roy K, Foote M. Trends Ecol Evol. 1997;12:277–281. doi: 10.1016/s0169-5347(97)81026-9. [DOI] [PubMed] [Google Scholar]
- 41.Stotz D F, Fitzpatrick J W, Parker T A, III, Moskovits D K. Neotropical Birds Ecology and Conservation. Chicago: University of Chicago Press; 1996. [Google Scholar]
- 42.Rylands A B, Mittermeier R A, Rodriguez-Luna E. Folia Primatol. 1997;68:134–160. [Google Scholar]
- 43.Fleagle J G, Reed K E. J Hum Evol. 1996;30:489–510. [Google Scholar]
- 44.Wright P C. In: Adaptive Radiations of Neotropical Primates. Norconk M, Rosenberger A, Garber P, editors. New York: Plenum; 1996. pp. 369–382. [Google Scholar]
- 45.Myers N. Science. 1997;278:597–598. [Google Scholar]
- 46.Hughes J B, Daily G C, Ehrlich P R. Science. 1997;278:689–692. doi: 10.1126/science.278.5338.689. [DOI] [PubMed] [Google Scholar]