Abstract
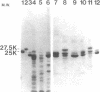
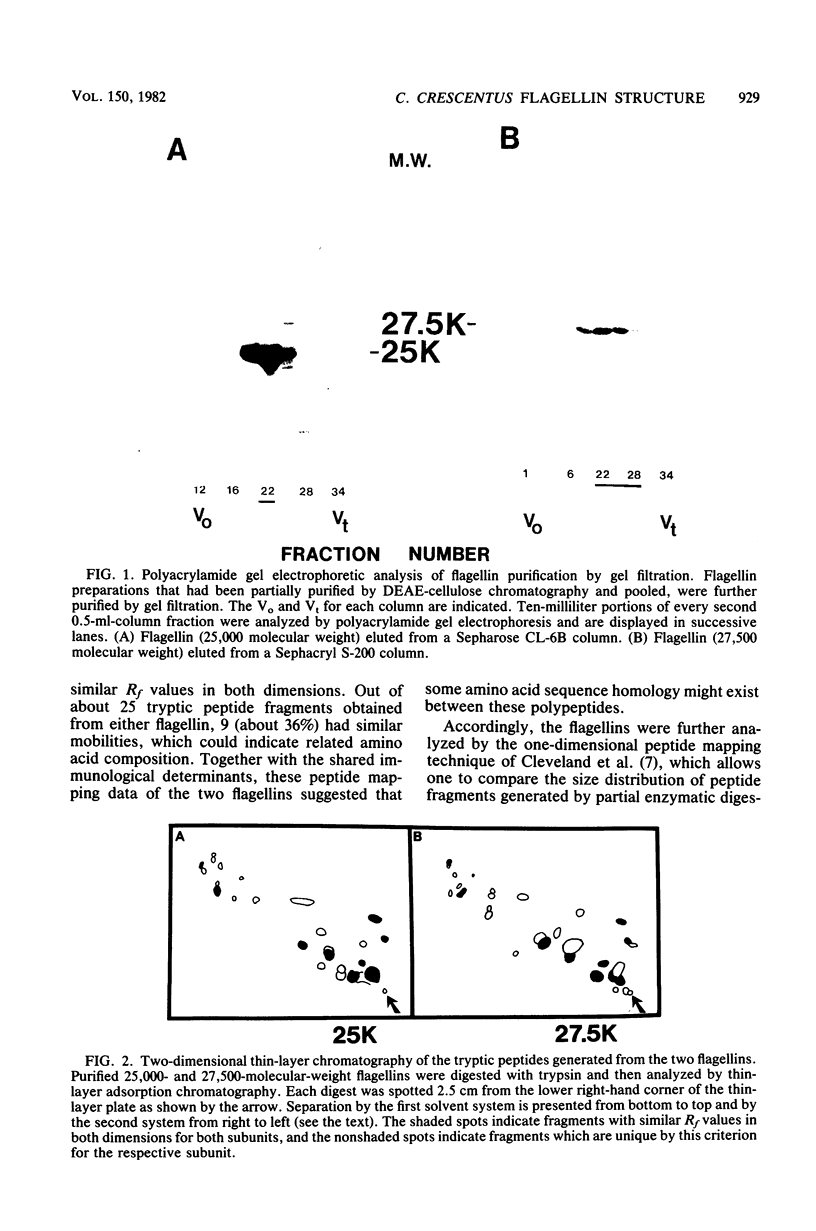
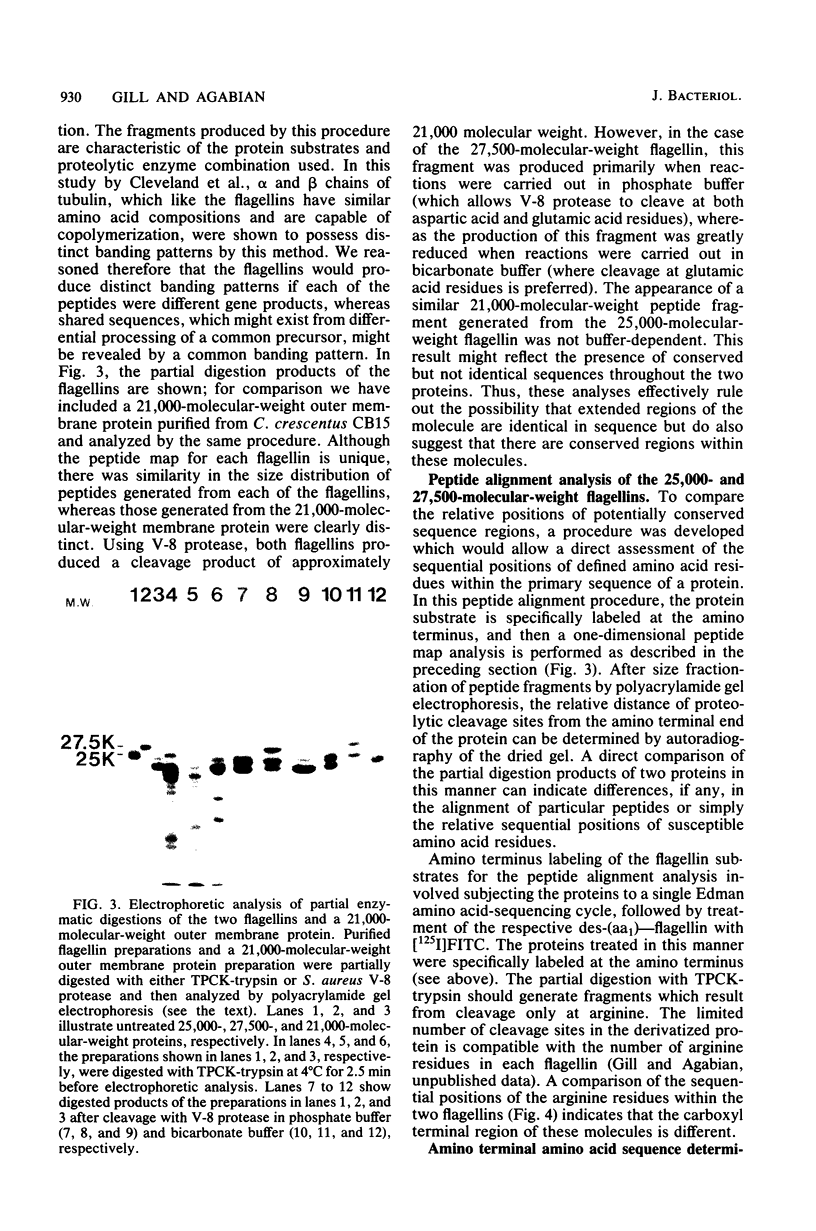
The flagellum of Caulobacter crescentus is composed of two flagellin polypeptide monomers which are distinguished by molecular weight and are closely related by biochemical and immunological criteria (C. Lagenaur and N. Agabian, J. Bacteriol. 132:731-733, 1977). The synthesis and assembly of these two flagellin proteins are developmentally regulated, and the periodicity of expression for each is distinct (C. Lagenaur and N. Agabian, J. Bacteriol. 135:1062-1069, 1978; M. A. Osley, M. Sheffery, and A. Newton, Cell 12:393-400, 1977). To understand the genetic and functional relationship between the 25,000- and 27,500-molecular-weight flagellins of C. crescentus CB15, a detailed comparative analysis of their protein structure was made, using a number of techniques, including one- and two-dimensional peptide mapping, a novel procedure of peptide alignment, and amino terminal amino acid sequence analysis. The tryptic peptides generated by each of the flagellins were compared by two-dimensional thin-layer chromatography. This peptide map analysis indicated that approximately 36% of the peptides generated from these two proteins had similar migration properties. Together with biochemical and immunological criteria, the two-dimensional peptide map suggested some structural relatedness between the monomers. However, a comparison of peptide fragments generated during partial protease digestion of each protein by a method of one-dimensional mapping indicated that the two proteins are structurally unique. A peptide alignment technique was developed to directly compare the primary structure of these proteins. In the peptide alignment procedure the amino terminus of each protein is radioactively labeled. After partial enzymatic digestion, the peptides are fractionated by polyacrylamide gel electrophoresis: those labeled at the amino terminus are then resolved by subsequent autoradiography. Each digest contains a family of amino-terminal-labeled fragments, the sizes of which reflect the sequential alignment of cleavage sites in the protein. A comparison of the alignment of specific cleavage sites of the two flagellins by this technique further established that each flagellin is structurally unique, particularly in the carboxyl terminal region. Finally, comparison of the amino terminal amino acid sequences indicated that the amino terminal region of both flagellins is highly conserved, but that the two polypeptides are clearly not identical. These findings strongly indicate that the two flagellins are encoded by distinct genetic loci and are not the product of novel processing of a single larger precursor.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N., Evinger M., Parker G. Generation of asymmetry during development. Segregation of type-specific proteins in Caulobacter. J Cell Biol. 1979 Apr;81(1):123–136. doi: 10.1083/jcb.81.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb D., Hofstee B. H. Gel isoelectric focusing for following the successive carbamylations of amino groups in chymotrypsinogen A. Anal Biochem. 1971 Mar;40(1):209–217. doi: 10.1016/0003-2697(71)90094-7. [DOI] [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Bridgen J. High sensitivity amino acid sequence determination. Application to proteins eluted from polyacrylamide gels. Biochemistry. 1976 Aug 10;15(16):3600–3604. doi: 10.1021/bi00661a030. [DOI] [PubMed] [Google Scholar]
- Bridgen P. J., Cross G. A., Bridgen J. N-terminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature. 1976 Oct 14;263(5578):613–614. doi: 10.1038/263613a0. [DOI] [PubMed] [Google Scholar]
- Calladine C. R. Design requirements for the construction of bacterial flagella. J Theor Biol. 1976 Apr;57(2):469–489. doi: 10.1016/0022-5193(76)90016-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cross G. A. Antigenic variation in trypanosomes. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):240–244. doi: 10.4269/ajtmh.1977.26.240. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Chang J. Y., Shaper J. H., Glazer A. N. Amino acid sequence of flagellin of Bacillus subtilis 168. III. Tryptic peptides, N-bromosuccinimide peptides, and the complete amino acid sequence. J Biol Chem. 1976 Feb 10;251(3):705–711. [PubMed] [Google Scholar]
- Fukuda A., Koyasu S., Okada Y. Characterization of two flagella-related proteins from Caulobacter crescentus. FEBS Lett. 1978 Nov 1;95(1):70–75. doi: 10.1016/0014-5793(78)80054-4. [DOI] [PubMed] [Google Scholar]
- Gabel C. A., Shapiro B. M. [125I]diiodofluorescein isothiocyanate: its synthesis and use as a reagent for labeling proteins and cells to high specific radioactivity. Anal Biochem. 1978 Jun 1;86(2):396–406. doi: 10.1016/0003-2697(78)90763-7. [DOI] [PubMed] [Google Scholar]
- Iino T. Genetics of structure and function of bacterial flagella. Annu Rev Genet. 1977;11:161–182. doi: 10.1146/annurev.ge.11.120177.001113. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979 Jan;137(1):627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Walsh M. P., Ely B., Shapiro L. Flagellar hook and basal complex of Caulobacter crescentus. J Bacteriol. 1979 Jun;138(3):984–989. doi: 10.1128/jb.138.3.984-989.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka M., Hayashi H. Studies on bacterial chemotaxis. I. Separation of proteins from cell envelope of Escherichia coli. J Biochem. 1977 Jul;82(1):87–54. doi: 10.1093/oxfordjournals.jbchem.a131696. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Caulobacter flagellar organelle: synthesis, compartmentation, and assembly. J Bacteriol. 1978 Sep;135(3):1062–1069. doi: 10.1128/jb.135.3.1062-1069.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Caulobacter flagellins. J Bacteriol. 1977 Nov;132(2):731–733. doi: 10.1128/jb.132.2.731-733.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Physical characterization of Caulobacter crescentus flagella. J Bacteriol. 1976 Oct;128(1):435–444. doi: 10.1128/jb.128.1.435-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., DeMartini M., Agabian N. Isolation and characterization of Caulobacter crescentus flagellar hooks. J Bacteriol. 1978 Nov;136(2):795–798. doi: 10.1128/jb.136.2.795-798.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- MacLeod A. R., Waterston R. H., Brenner S. An internal deletion mutant of a myosin heavy chain in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5336–5340. doi: 10.1073/pnas.74.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H., Ishida N., Kawauchi H., Tsujimura K. Reaction of fluorescein-isothiocyanate with proteins and amino acids. I. Covalent and non-covalent binding of fluorescein-isothiocyanate and fluorescein to proteins. J Biochem. 1969 May;65(5):777–783. doi: 10.1093/oxfordjournals.jbchem.a129077. [DOI] [PubMed] [Google Scholar]
- Marie J., Simon M. P., Dreyfus J. C., Kahn A. One gene, but two messenger RNAs encode liver L and red cell L' pyruvate kinase subunits. Nature. 1981 Jul 2;292(5818):70–72. doi: 10.1038/292070a0. [DOI] [PubMed] [Google Scholar]
- Marino W., Ammer S., Shapiro L. Conditional surface structure mutants of Caulobacter crescentus temperature-sensitive flagella formation due to an altered flagellin monomer. J Mol Biol. 1976 Oct 25;107(2):115–130. doi: 10.1016/s0022-2836(76)80021-6. [DOI] [PubMed] [Google Scholar]
- Osley M. A., Sheffery M., Newton A. Regulation of flagellin synthesis in the cell cycle of caulobacter: dependence on DNA replication. Cell. 1977 Oct;12(2):393–400. doi: 10.1016/0092-8674(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Reznikoff W. S. The functional differences in the inverted repeats of Tn5 are caused by a single base pair nonhomology. Cell. 1981 Jan;23(1):191–199. doi: 10.1016/0092-8674(81)90284-1. [DOI] [PubMed] [Google Scholar]
- Simon M., Zieg J., Silverman M., Mandel G., Doolittle R. Phase variation: evolution of a controlling element. Science. 1980 Sep 19;209(4463):1370–1374. doi: 10.1126/science.6251543. [DOI] [PubMed] [Google Scholar]
- Smit J., Hermodson M., Agabian N. Caulobacter crescentus pilin. Purification, chemical characterization, and NH2-terminal amino acid sequence of a structural protein regulated during development. J Biol Chem. 1981 Mar 25;256(6):3092–3097. [PubMed] [Google Scholar]
- Tronick S. R., Martinez R. J. Methylation of the flagellin of Salmonella typhimurium. J Bacteriol. 1971 Jan;105(1):211–219. doi: 10.1128/jb.105.1.211-219.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- West D., Lagenaur C., Agabian N. Isolation and characterization of Caulobacter crecentus bacteriophage phi Cd1. J Virol. 1976 Feb;17(2):568–575. doi: 10.1128/jvi.17.2.568-575.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]