Figure 3.
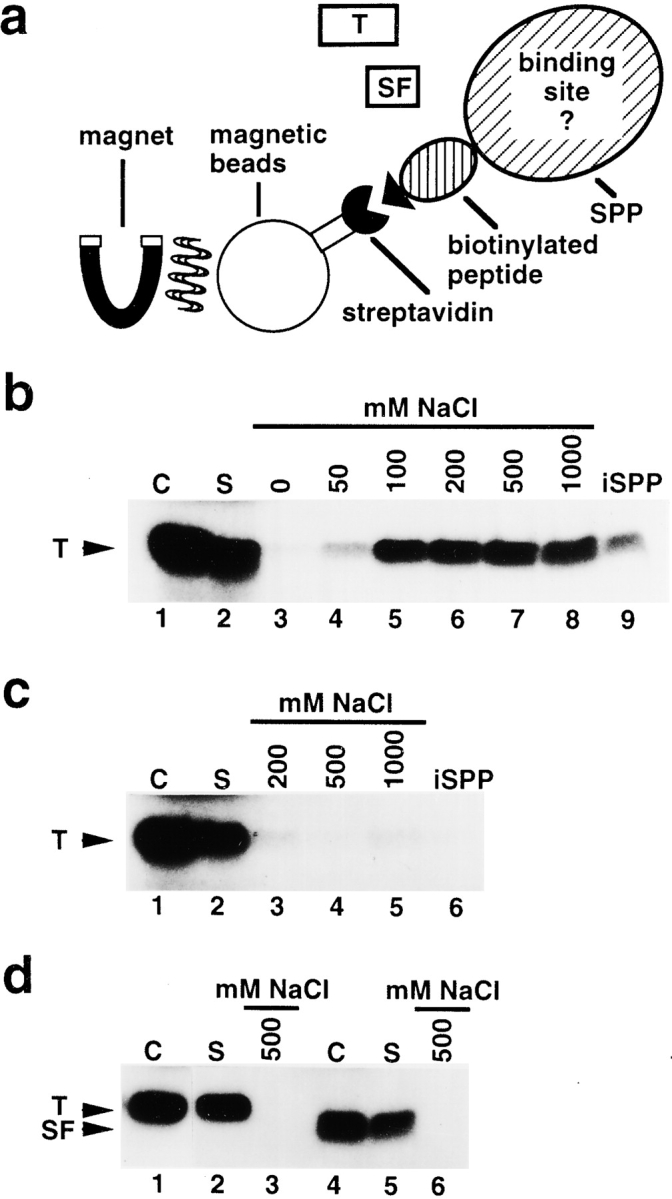
SPP contains a binding site for the intact transit peptide. a, Schematic representation of the binding assay (see Materials and Methods). Recombinant SPP was immobilized onto streptavidin-coated magnetic beads that bound to its biotin-containing peptide tag. [35S]methionine-labeled preparations of FD transit peptide (T) or its subfragment (SF) were added. Using a magnet, the supernatant containing the unbound substrate was separated from the immobilized SPP fraction with the bound substrate. b, Binding of FD transit peptide to immobilized SPP. Total amount of substrate used in one reaction, control lane 1; supernatant containing unbound substrate, lane 2; bound substrate independently eluted at NaCl concentrations between 0 and 1,000 mM, lanes 3–8; substrate still bound to immobilized SPP after washing at 1,000 mM NaCl, lane 9. c, Binding of FD transit peptide to immobilized SPP in the presence of 5 mM 1,10-phenanthroline. Substrate, control lane 1; supernatant, lane 2; bound substrate independently eluted at NaCl concentrations of 200, 500, and 1,000 mM, lanes 3–5; substrate bound to immobilized SPP after washing at 1,000 mM NaCl, lane 6. d, Binding of FD transit peptide to streptavidin-coated magnetic beads incubated with extract of E. coli cells. Substrate, control lane 1; supernatant, lane 2; bound substrate eluted from the magnetic beads fraction at 500 mM NaCl, lane 3. Binding of subfragment of FD transit peptide to immobilized SPP. Substrate, control lane 4; supernatant, lane 5; bound substrate eluted from immobilized SPP at 500 mM NaCl, lane 6.
