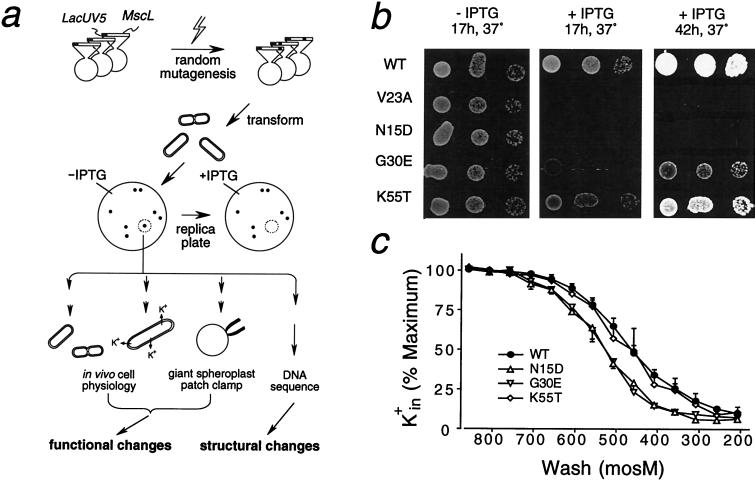
Figure 1.
(a) The experimental scheme. A plasmid with an inducible Lac promoter and the mscL coding region was mutagenized in vitro and used to transform mscL-null E. coli. Transformant colonies whose replicas failed to appear after induction (+IPTG) were isolated. We then determined their growth rates, K+ contents, and MS conductances as well as the mutated DNA sequence so as to correlate functional with structural changes. (b) Growth on Luria–Bertani nutrient plates. Each row shows the growth pattern of three 10-μl drops of inoculate from cultures of 0.3 OD650 diluted 104, 105, and 106 fold. (Left) Plates without IPTG where all strains grew normally. (Center) With 1 mM IPTG, only the wild type (WT) grew normally. (Right) Same plate as center but photographed much later and at a higher contrast. The last two panels together show that induced K55T colonies grew slowly, G30E even more slowly, and N15D or V23A not at all on plates. (Induced N15D grew very slowly in liquid media, but V23A not at all; see Table 1) (c) Loss of cell K+ on osmotic downshock. One hour after induction, bacteria were downshocked from 858 milliosmol to different hypo-osmolarity (abscissa) before cell K+ was determined by flame photometry and plotted as percentage of the induced but no-shock control (mean ± SEM, n = 3–8). N15D (severe) and G30E (milder mutant) but not K55T (mildest) mutant consistently lose more K+ at milder downshocks. V23A (very severe) has a low viability and was not tested.