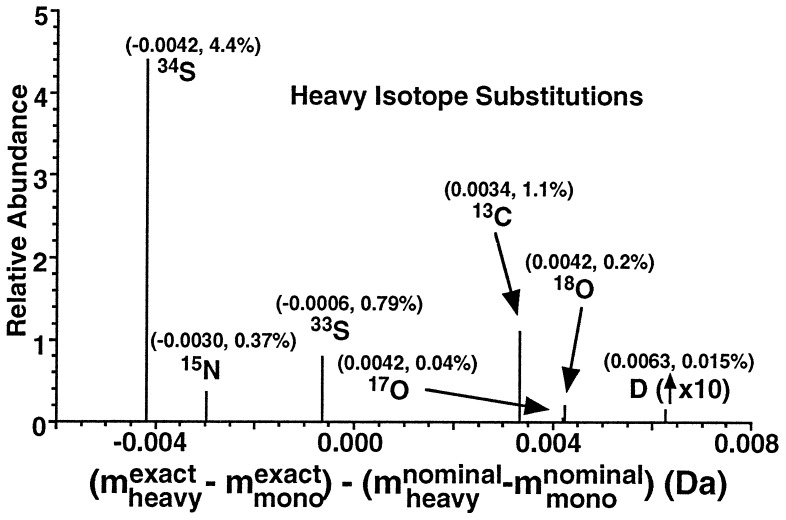
Figure 4.
Relative abundance (ordinate) and mass difference (abscissa) resulting from substitution of a single heavy isotope for the most abundant (lower-mass) isotope of that element. (The abundance for deuterium is magnified by a factor of 10 to make it more visible.) For ease in computation, each mass difference has been adjusted for the nominal mass difference between the two isotopes. Each displayed mass difference is thus like a mass defect (the difference between exact and nominal mass), in the sense that the mass shift is a small fraction of 1 Da. These values facilitate the identification (by abundance and accurate mass) of various fine structure components at a given nominal mass (see text).