Abstract
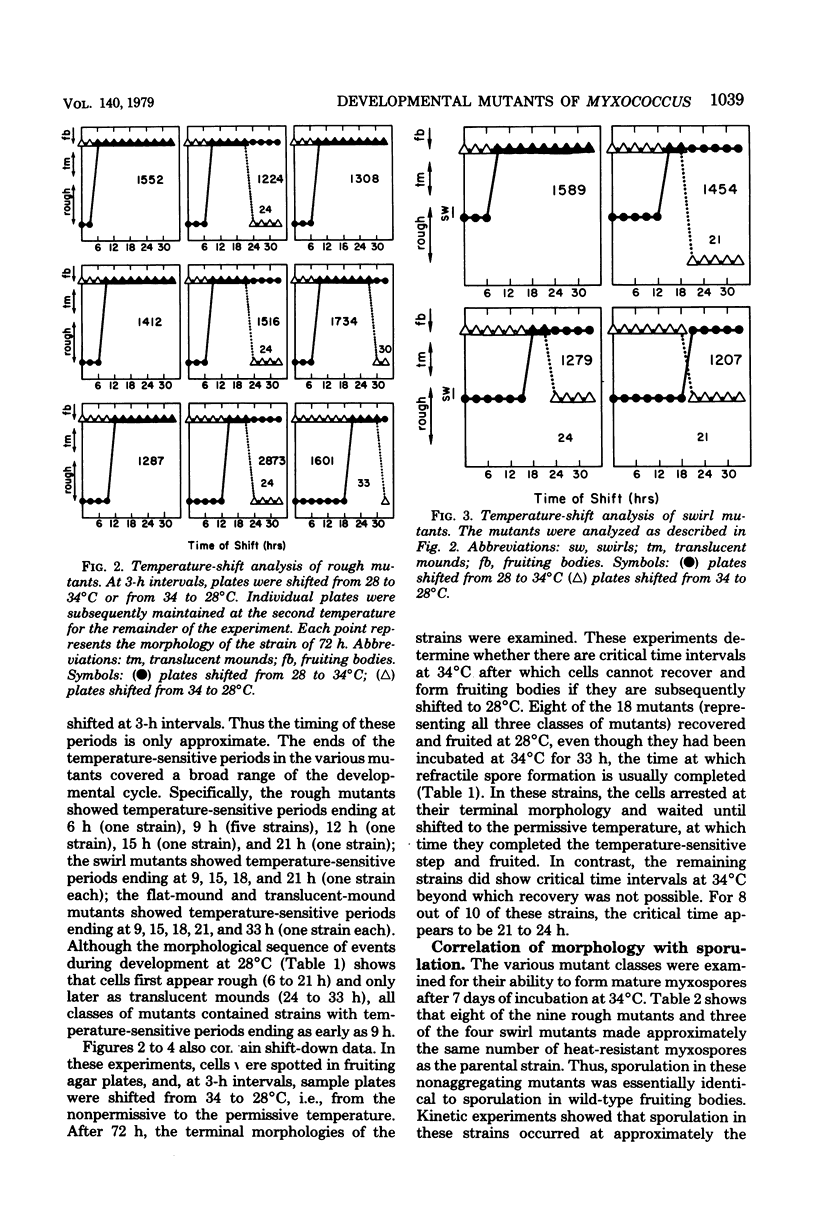
Fruiting-body formation in the bacterium Myxococcus xanthus consists of a temporal sequence of cellular aggregation and sporulation. To examine the developmental stages more closely, we established synchronous and reproducible conditions for fruiting-body formation. Mutants that are temperature sensitive for fruiting-body formation were isolated and analyzed under these conditions. The terminal morphologies of the mutant strains at the nonpermissive temperature were found to resemble intermediate stages of fruiting-body formation and therefore were grouped in the following phenotypic classes: (i) rough mutants, which show no aggregation; (ii) swirl mutants, which show defective aggregation; (iii) flat-mound mutants and translucent-mound mutants, mutants which aggregate but show very low levels of sporulation. The mutants were characterized by temperature-shift experiments and found to exhibit discrete and reproducible temperature-sensitive periods. The ends of the temperature-sensitive periods in the various mutants covered a broad range of the developmental cycle. No correlation was found between the terminal morphologies at the restrictive temperature and the timing of the temperature-sensitive periods. However, the terminal morphologies correlated well with sporulation. The rough and swirl mutants produced normal numbers of myxospores at 34°C even though they failed to aggregate. In contrast, the flat-mound and translucent-mound mutants, which aggregate normally, produced very few spores. The translucent-mound mutants were also temperature sensitive for induction of glycerol spores. The results indicate that both aggregation and sporulation are initiated early in the developmental cycle and that these processes are largely independent of each other.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon K., Clutter D., Kottel R. H., Orlowski M., White D. Carbohydrate accumulation during myxospore formation in Myxococcus xanthus. J Bacteriol. 1975 Dec;124(3):1635–1636. doi: 10.1128/jb.124.3.1635-1636.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. P., Kaiser D. Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J Bacteriol. 1978 Feb;133(2):763–768. doi: 10.1128/jb.133.2.763-768.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard R. P., Dworkin M. A bacteriophage for Myxococcus xanthus: isolation, characterization and relation of infectivity to host morphogenesis. J Bacteriol. 1966 Mar;91(3):1305–1313. doi: 10.1128/jb.91.3.1305-1313.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J. M., Geisselsoder J., Zusman D. R. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978 Feb 25;119(2):167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- Campos J. M., Zusman D. R. Regulation of development in Myxococcus xanthus: effect of 3':5'-cyclic AMP, ADP, and nutrition. Proc Natl Acad Sci U S A. 1975 Feb;72(2):518–522. doi: 10.1073/pnas.72.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- Hagen D. C., Bretscher A. P., Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978 Jun;64(2):284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Inouye M., Inouye S., Zusman D. R. Biosynthesis and self-assembly of protein S, a development-specific protein of Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):209–213. doi: 10.1073/pnas.76.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Inouye S., Zusman D. R. Gene expression during development of Myxococcus xanthus: pattern of protein synthesis. Dev Biol. 1979 Feb;68(2):579–591. doi: 10.1016/0012-1606(79)90228-8. [DOI] [PubMed] [Google Scholar]
- MCVITTIE A., MESSIK F., ZAHLER S. A. Developmental biology of Myxococcus. J Bacteriol. 1962 Sep;84:546–551. doi: 10.1128/jb.84.3.546-551.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd K., Zusman D. R. Rifampin-resistant mutants of Myxococcus xanthus defective in development. J Bacteriol. 1979 Jan;137(1):295–300. doi: 10.1128/jb.137.1.295-300.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wireman J. W., Dworkin M. Morphogenesis and developmental interactions in myxobacteria. Science. 1975 Aug 15;189(4202):516–523. doi: 10.1126/science.806967. [DOI] [PubMed] [Google Scholar]



