Abstract
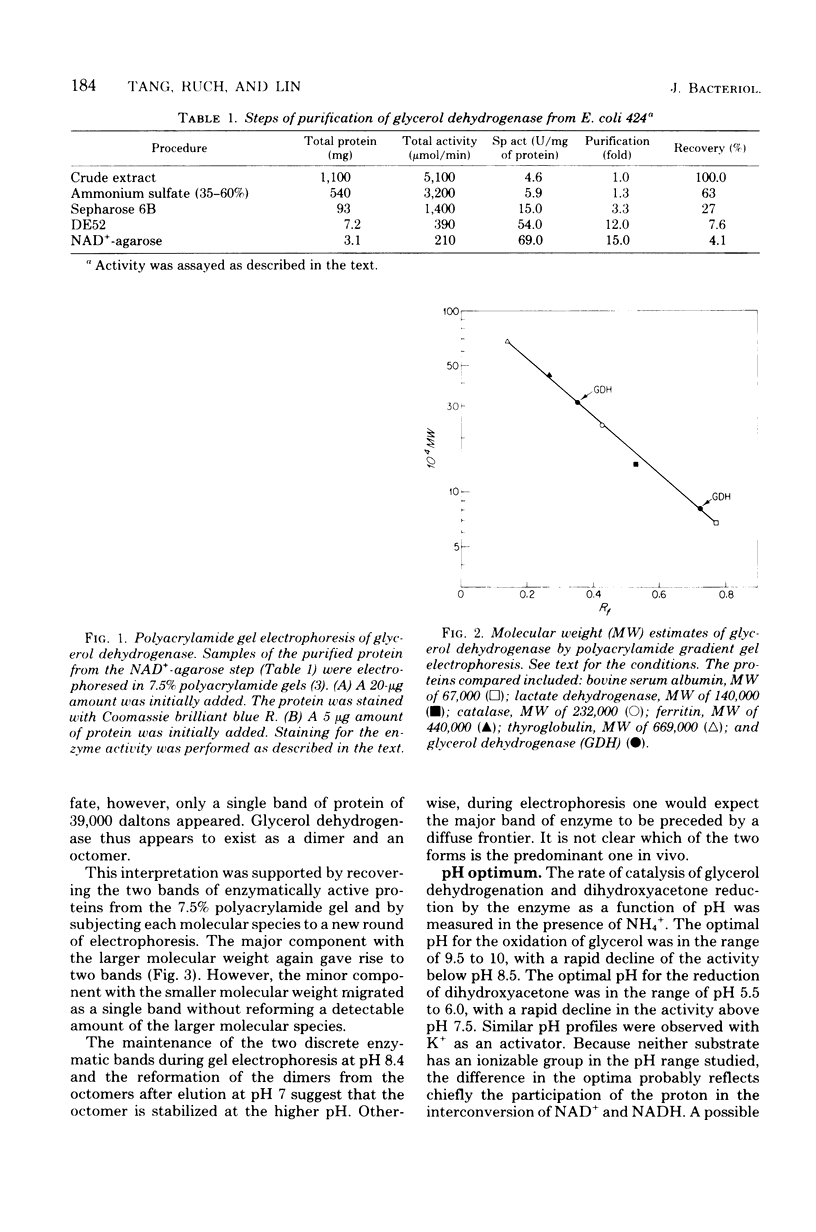
Glycerol:NAD+2-OXIDOREDUCTASE (EC 1.1.1.6) was purified to homogeneity from a mutant of Escherichia coli K12 that uses this enzyme, instead of ATP:glycerol 3-phosphotransferase (EC 2.7.1.30), as the first enzyme for the dissimilation of glycerol. Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate shows a subunit of 39,000 daltons. During electrophoresis under nondenaturing conditions, the protein migrates as two bands. These two forms, both of which are enzymatically active, appear to be dimers and octomers of the same subunit. The optimal pH for the oxidation of glycerol is about 10, and that for the reduction of dihydroxyacetone is about 6. Glycerol dehydrogenation is highly activated by NH4+, K+, or Rb+, but strongly inhibited by N-ethylmalemide, 8-hydroxyquinoline, 1,10-phenanthroline, Cu2+, and Ca2+. The enzyme exhibits a broad substrate specificity. In addition to glycerol, it act on 1,2-propanediol and several of its analogs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASNIS R. E., BRODIE A. F. A glycerol dehydrogenase from Escherichia coli. J Biol Chem. 1953 Jul;203(1):153–159. [PubMed] [Google Scholar]
- Cocks G. T., Aguilar T., Lin E. C. Evolution of L-1, 2-propanediol catabolism in Escherichia coli by recruitment of enzymes for L-fucose and L-lactate metabolism. J Bacteriol. 1974 Apr;118(1):83–88. doi: 10.1128/jb.118.1.83-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERNER S. A., WU T. T., LIN E. C. EVOLUTION OF A CATABOLIC PATHWAY IN BACTERIA. Science. 1964 Dec 4;146(3649):1313–1315. doi: 10.1126/science.146.3649.1313. [DOI] [PubMed] [Google Scholar]
- LIN E. C., MAGASANIK B. The activation of glycerol dehydrogenase from Aerobacter aerogenes by monovalent cations. J Biol Chem. 1960 Jun;235:1820–1823. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Mortlock R. P., Fossitt D. D., Wood W. A. A basis for utlization of unnatural pentoses and pentitols by Aerobacter aerogenes. Proc Natl Acad Sci U S A. 1965 Aug;54(2):572–579. doi: 10.1073/pnas.54.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch F. E., Lengeler J., Lin E. C. Regulation of glycerol catabolism in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):50–56. doi: 10.1128/jb.119.1.50-56.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T., Chused T. M., Lin E. C. Ferrous-activated nicotinamide adenine dinucleotide-linked dehydrogenase from a mutant of Escherichia coli capable of growth on 1, 2-propanediol. J Bacteriol. 1969 Apr;98(1):87–95. doi: 10.1128/jb.98.1.87-95.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin E. J., Freedberg W. B., Lin E. C. Kinase replacement by a dehydrogenase for Escherichia coli glycerol utilization. J Bacteriol. 1977 Sep;131(3):1026–1028. doi: 10.1128/jb.131.3.1026-1028.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. S., Rigby P. W., Hartley B. S. Ribitol dehydrogenase from Klebsiella aerogenes. Purification and subunit structure. Biochem J. 1974 Sep;141(3):693–700. doi: 10.1042/bj1410693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]




