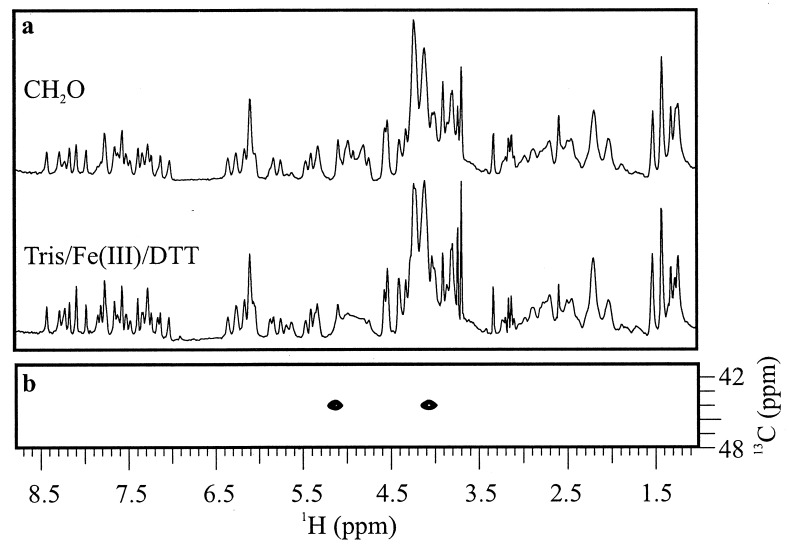
Figure 2.
The 500-MHz 1H and HSQC NMR spectra of covalent Adriamycin-DNA adducts at 10°C. (a) Comparison of CH2O- and Tris/Fe(III)/DTT-mediated Adriamycin-DNA adducts by 1H NMR indicates that the two are structurally equivalent. (b) Portion of the carbon HSQC spectrum of 13CH2O-mediated Adriamycin-DNA. The methylene 13C has strong cross-peaks to each of its nonequivalent geminal protons, which in turn bear cross-peak arrays structurally consistent with the incorporation of CH2O into the Adriamycin-DNA adduct as shown in Fig. 1.