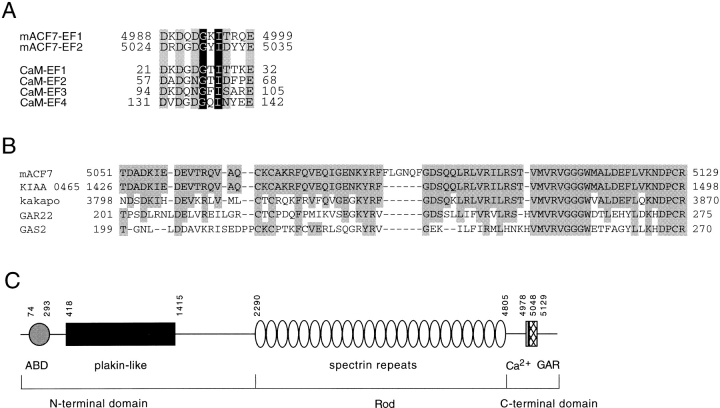
Figure 2.
Sequence analysis of mACF7 COOH-terminal domain. (A) The two putative EF-hand calcium binding motifs of mACF7 are compared with those of mouse calmodulin (CaM). The amino acids that contain side chains with oxygen atoms for Ca2+ binding are shaded in gray, whereas the highly conserved glycine and hydrophobic amino acid are shaded in black with white letters. (B) Sequence comparison of mACF7 GAR region, human KIAA 0465, Drosophila Kakapo, human GAR22, and human GAS2 protein. The identical amino acids are shaded. (C) Schematic representation of the domain structure of mACF7. The NH2-terminal head domain consists of an ABD and a plakin-like globular domain. The rod domain is composed of 23 dystrophin-like spectrin repeats. The COOH-terminal tail domain is composed of two EF-hand motifs (Ca2+) and a Gas2/GAR22 homology region (GAR). The number of the amino acids that marked the boundary of each domain is also indicated.