Abstract
We describe here a rapid and semiautomated method for the determination of rubella virus immunoglobulin G (IgG) avidity with the VIDAS instrument. A total of 153 serum samples from persons with naturally acquired rubella virus infections (n = 98), from vaccinated persons (n = 44), and from patients with autoantibodies (n = 11) were included in this study. The rubella virus-specific IgG avidity assay we developed for the VIDAS instrument was evaluated by comparison with an in-house method. Results obtained with the VIDAS instrument allow considering this method valuable to help confirm or exclude acute primary infection or recent vaccination.
Thanks to extensive vaccination programs, rubella virus infection has dramatically decreased, especially in developed countries. However, rubella vaccine coverage is not sufficient throughout the world and rubella cases are still reported. As clinical diagnosis is unreliable, laboratory diagnosis is necessary to confirm acute rubella virus infection. This diagnosis is based on the observation of seroconversion or on the detection of both rubella virus-specific immunoglobulin G (RV-IgG) and RV-IgM. Seroconversion is rarely observed and is not sufficient to confirm acute rubella virus infection. Indeed, as the cutoff of rubella tests is relatively high (10 IU/ml or 15 IU/ml), the first serum sample tested can be considered negative for rubella virus antibodies whereas it may contain trace amounts (below the cutoff) of RV-IgG. Under these conditions, “seroconversion” cannot always be related to acute rubella virus infection. In the same way, if RV-IgM is always detected in acute rubella virus infection, it can also be detected for a long time, especially after vaccination, because of polyclonal stimulation of the immune system and also because of reinfection (1, 2, 6, 12, 13, 14). Among the supplementary tests used to confirm recent primary rubella virus infection, RV-IgG avidity have proved to be very helpful (3, 4, 5, 7, 8). Recently, commercial RV-IgG avidity assays have been compared (11). Among the five assays tested, only the Euroimmun and Radim rubella virus IgG avidity assays performed well, demonstrating excellent correlation with the “gold standard” and for this reason were the only ones that were considered reliable. These commercial tests are processed in microplates and need more than 1 h to be completed. The aim of our study was to develop a rapid and semiautomated RV-IgG avidity method for the VIDAS instrument (bioMérieux, Marcy-l'Étoile, France) that allows single-dose testing.
MATERIALS AND METHODS
Serum samples.
A total of 153 serum samples were included in this RV-IgG avidity study. Ninety-eight samples were collected after naturally acquired rubella virus infection; 19 were from recently acquired infections and were collected between the onset of infection and 1 month after, 5 were collected between 1 and 2 months after the onset of infection, and 74 were collected more than 3 months after the onset of infection (50 were RV-IgG positive and RV-IgM negative, and 24 were RV-IgG positive and RV-IgM positive or equivocal with high RV-IgG avidity). Among these samples, 14 were from five patients and were collected between the onset of infection and less than 2 months after (follow-up). For all of the patients but two with primary infections, the exposure date was estimated according to the date a rash appeared. For the other two primary infections, which occurred in symptom-free patients, the exposure date was estimated according to the value of the RV-IgG avidity index. Forty-four samples were collected after rubella vaccination; 11 were collected between vaccination and 1 month after, 7 were collected between 1 and 2 months after vaccination, 10 were collected between 2 and 3 months after vaccination, and 16 were collected more than 3 months after vaccination. Among these samples, 30 were from 8 patients and were collected between vaccination and up to 176 days after (follow-up). Eleven samples were from patients with high titers of autoantibodies (five rheumatoid factor-positive serum samples [latex agglutination assay titers of >320 by Rhumalatex; Sofibel, Levallois-Perret, France] and six anti-nuclear antibody-positive serum samples [indirect immunofluorescence assay titers of >1,280; Bio-Rad, Marnes-la-Coquette, France]). All of the serum samples but two from naturally acquired infection and vaccination were collected from pregnant women referred to our laboratory for rubella virus antibody screening or for additional testing when RV-IgM was detected.
RV-IgG and RV-IgM assays.
RV-IgG was measured with the VIDAS RUB IgG II assay (cutoff, 15 IU/ml), and RV-IgM was measured with the VIDAS RUB IgM assay (cutoff index, 1.2; gray zone, 0.8 to <1.2) (bioMérieux, Marcy l'Étoile, France).
RV-IgG avidity assays.
The RV-IgG avidity assay that we developed for the VIDAS instrument was compared with an in-house assay. Briefly, the latter method is based on the use of the Enzygnost anti-RV-IgG kit (Dade Behring, Marburg, Germany) according to the manufacturer's instructions, except for the wash step after the first antibody incubation, when 6 M urea is added in parallel with phosphate-buffered saline-Tween 20, as previously described (10, 15). Results are expressed as ratios of absorbance values for single serum dilutions with and without a denaturing agent. An avidity index of <30% is considered very low, one between 30% and 70% is considered low, one between 70% and 90% is considered moderate, and one of >90% is considered high.
The VIDAS RUB IgG II assay is an automated enzyme-linked fluorescent immunoassay. A pipette tip-like disposable device coated with inactivated rubella virus constitutes the solid phase and serves as the pipettor. All of the other reagents (mouse monoclonal anti-human IgG antibodies labeled with alkaline phosphatase, washing buffers, and substrate) are presented in a 10-well foil-sealed strip. The test is performed by addition of the specimen to the first well. The results are expressed as a relative fluorescence value (RFV) and as the number of VIDAS international units per milliliter. For the determination of avidity, two VIDAS RUB IgG II tests were used. One test served as the reference. In the other, the wash buffer in well 4 of the strip was replaced with a buffer containing 6 M urea. The avidity index was determined by calculating the ratio of the RFV obtained with the reference strip to the RFV obtained with the strip containing urea.
Precision studies. (i) Intra-assay precision.
Two different tests were performed to check intra-assay precision. In one, 1 serum sample with low RV-IgG avidity and 1 serum sample with high RV-IgG avidity were tested three times with the VIDAS instrument, and in the other, 10 serum samples with high RV-IgG avidity were tested twice with each avidity assay (in-house and VIDAS assays).
(ii) Interassay precision.
To test interassay precision, one serum sample with low RV-IgG avidity and one serum sample with high RV-IgG avidity were tested once every day for 3 consecutive days with the VIDAS instrument.
Dilution study.
Twofold serial dilutions of three serum samples with IgG levels between 133 IU/ml and 309 IU/ml were tested for RV-IgG and RV-IgG avidity with the VIDAS instrument. Dilutions were performed with RV-IgG-negative serum samples in order to maintain a physiological protein concentration.
Statistical analysis.
The statistical significance of RV-IgG avidity measured by the two techniques was analyzed with the Student test. A P value of <0.01 was accepted as statistically significant.
RESULTS
Precision study.
In the intra-assay precision study, the coefficients of variation (CVs) were found to be 3.95% and 4.14% for low-avidity and high-avidity samples, respectively, with the VIDAS instrument. For the 10 serum samples analyzed by the in-house technique, the CVs ranged from 0.7% to 6.8% and with the VIDAS assay, they ranged from 0.9% to 4.4%, except for 1 serum sample (CV, 13.4%).
In the interassay precision study, the CVs were found to be 4.87% and 3.53% for low- and high-avidity samples, respectively, by the VIDAS assay.
Dilution study.
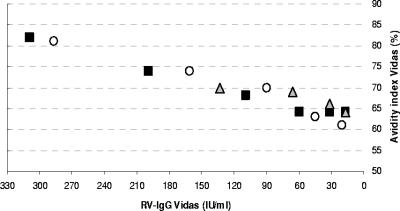
For serially diluted RV-IgG-negative serum samples A, B, and C, the avidity results decreased from 82%, 81%, and 70% (RV-IgG results were as follows: sample A, 309 IU/ml; sample B, 287 IU/ml; sample C, 133 IU/ml) to 64%, 61%, and 64%, respectively (RV-IgG results near the cutoff) (Fig. 1).
FIG. 1.
Dilution assays. Symbols: circles, serum sample A; squares, serum sample B; triangles, serum sample C.
RV-IgG avidity results for serum samples collected after naturally acquired infection.
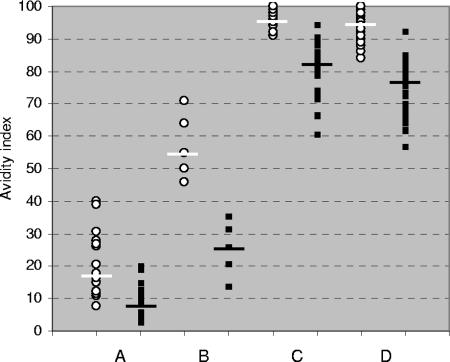
The RV-IgG results obtained for all of the samples from naturally acquired infections are summarized in Fig. 2. The results obtained with 15 RV-IgG-positive serum samples collected up to 1 month after exposure to rubella virus were as follows: in-house avidity results ranged from 8% to 40% (median, 18%), and VIDAS avidity results ranged from 3% to 20% (median, 9%) (P < 0.01). All of the VIDAS avidity results but two were found to be lower than the in-house results. For serum samples taken between 1 and 2 months after exposure, the avidity indexes obtained by the in-house method ranged from 46% to 71% (median, 55%) and the avidity indexes obtained by the VIDAS assay ranged from 14% to 36% (median, 26%) (P < 0.01). Concerning past rubella virus infections (samples collected more than 3 months after the onset of infection), RV-IgG levels of the 24 IgG-positive, IgM-positive, high-RV-IgG-avidity serum samples ranged from 68 IU/ml to 1,944 IU/ml, and RV-IgM indexes ranged from 0.85 to 14.84. Results obtained by the in-house avidity technique ranged from 91% to 100% (median, 96%), and those obtained with the VIDAS assay ranged from 61% to 95% (median, 82%) (P < 0.001). RV-IgG levels of the 50 IgG-positive, IgM-negative serum samples ranged from 42 IU/ml to 858 IU/ml. The results obtained by the in-house avidity technique ranged from 84% to 100% (median, 95%), and those obtained with the VIDAS assay ranged from 57% to 93% (median, 77%) (P < 0.001).
FIG. 2.
Comparison of avidity indexes obtained by the in-house method (circles) and those obtained by the VIDAS assay (squares) following naturally acquired rubella virus infection. A, less than 1 month after exposure (n = 15); B, 1 to 2 months after exposure (n = 5); C, more than 3 months after exposure (RV-IgG positive, RV-IgM positive or equivocal, and high RV-IgG avidity; n = 24); D, more than 3 months after exposure (RV-IgG positive, RV-IgM negative; n = 50). Medians are indicated.
The RV-IgG avidity indexes obtained with the in-house and VIDAS assays at different intervals of time after primary infection (follow-up) are shown in Table 1. For 9 out of 10 RV-IgG-positive samples, the avidity indexes found with the VIDAS assay were lower than those found with the in-house assay. Furthermore, the values obtained with the two assays were found to be parallel for three out of the four patients with sequential serum samples (patients 1, 2, and 3).
TABLE 1.
Follow-up of primary infections
| Patient and no. of days postexposure | RV-IgM index | RV-IgG IU/ml | RV-IgG avidity index (%)
|
|
|---|---|---|---|---|
| In-house method | VIDAS assay | |||
| 1 | ||||
| 15 | 0.15 | 2 | NDa | ND |
| 31 | 10.75 | 230 | 39 | 15 |
| 52 | 3.75 | 236 | 64 | 36 |
| 2 | ||||
| Ub | 8.39 | 210 | 32 | 19 |
| U | 3.15 | 233 | 50 | 34 |
| 3 | ||||
| 15 | 3.29 | 3 | ND | ND |
| 26 | 8.53 | 253 | 40 | 9 |
| 34 | 6.85 | 292 | 46 | 14 |
| 48 | 4.64 | 333 | 71 | 32 |
| 4 | ||||
| 15 | 9.13 | 18 | ND | ND |
| 43 | 14.7 | 106 | 50 | 21 |
| 5 | ||||
| 15 | 0.15 | 2 | ND | ND |
| 18 | 2.3 | 24 | 12 | 20 |
| 39 | 5.89 | 277 | 55 | 26 |
ND, not done.
U, unknown.
RV-IgG results for serum samples collected after vaccination.
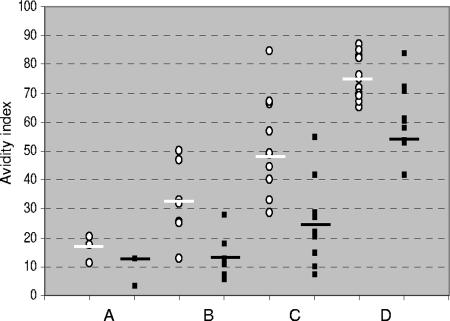
The RV-IgG avidity results obtained with serum samples collected after vaccination are summarized in Fig. 3. The results obtained with 3 RV-IgG-positive serum samples out of the 11 serum samples collected up to 1 month after vaccination were as follows: the in-house avidity results ranged from 11% to 20% (median, 18%), and the VIDAS avidity results ranged from 3% to 13% (median, 13%). For the seven serum samples taken between 1 and 2 months after vaccination, the avidity indexes obtained by the in-house method ranged from 13% to 50% (median, 32%) and the avidity indexes measured with the VIDAS assay ranged from 6% to 18% (median, 13%) (P < 0.001). For the 10 serum samples taken between 2 and 3 months after vaccination, the avidity indexes obtained with the in-house method ranged from 29% to 85% (median, 49%) and the avidity indexes measured with the VIDAS instrument ranged from 7% to 55% (median, 25%) (P < 0.01). For the 16 serum samples collected more than 3 months after vaccination, the in-house avidity indexes ranged from 65% to 87% (median, 76%) and the VIDAS results ranged from 42% to 83% (median, 54%) (P < 0.001). The VIDAS avidity results found were always lower, except in three cases.
FIG. 3.
Comparison of avidity indexes obtained with the in-house method (circles) and those obtained with the VIDAS assay (squares) following rubella vaccination. A, less than 1 month after vaccination (n = 3); B, 1 to 2 months after vaccination (n = 7); C, 2 to 3 months after vaccination (n = 10); D, more than 3 months after vaccination (n = 16). Medians are indicated.
The RV-IgG avidity indexes obtained with the in-house and VIDAS assays at different intervals of time after vaccination (follow-up) are shown in Table 2. For the 22 sequential RV-IgG-positive serum samples collected up to 176 days after vaccination, the VIDAS avidity results were always lower than the in-house results, except in one case.
TABLE 2.
Follow-up of vaccinations
| Patient and no. of days postvaccination | RV-IgM index | RV-IgG IU/ml | RV-IgG avidity index (%)
|
|
|---|---|---|---|---|
| In-house method | VIDAS assay | |||
| 1 | ||||
| 0 | 0.24 | 1 | NDa | ND |
| 20 | 1.51 | 8 | ND | ND |
| 34 | 7.22 | 42 | 13 | 6 |
| 62 | 3 | 103 | 49 | 27 |
| 89 | 1.63 | 113 | 57 | 29 |
| 2 | ||||
| 0 | 0.08 | 1 | ND | ND |
| 24 | 7.3 | 12 | ND | ND |
| 68 | 3.33 | 44 | 29 | 10 |
| 81 | 1.98 | 64 | 45 | 22 |
| 3 | ||||
| 0 | 0.64 | 1 | ND | ND |
| 18 | 5.79 | 30 | 20 | 13 |
| 34 | 4.79 | 43 | 33 | 18 |
| 90 | 11.8 | 127 | 85 | 55 |
| 4 | ||||
| 0 | 0.1 | 0 | ND | ND |
| 38 | 7.42 | 66 | 32 | 13 |
| 51 | 4.49 | 77 | 47 | 18 |
| 66 | 3.04 | 93 | 66 | 29 |
| 94 | 1.62 | 118 | 75 | 42 |
| 5 | ||||
| 0 | 0.1 | 0 | ND | ND |
| 27 | 10.21 | 26 | 11 | 13 |
| 47 | 4.3 | 55 | 25 | 11 |
| 73 | 1.71 | 75 | 49 | 21 |
| 6 | ||||
| 18 | 1.64 | 7 | ND | ND |
| 22 | 7.12 | 51 | 18 | 3 |
| 31 | 3.6 | 88 | 25 | 7 |
| 46 | 1.1 | 84 | 40 | 15 |
| 7 | ||||
| 60 | 1.66 | 134 | 50 | 28 |
| 90 | 0.86 | 106 | 67 | 42 |
| 8 | ||||
| 150 | 3.49 | 169 | 65 | 50 |
| 176 | 2.86 | 157 | 72 | 53 |
ND, not done.
Interference study.
Among the 11 serum samples with autoantibodies, only 1 was RV-IgG negative with the Enzygnost anti-RV-IgG and VIDAS RUB IgG II assays. No absorbance value was detected in the well coated with noninfected cells (control antigen) for the 10 RV-IgG-positive samples.
DISCUSSION
Commercial and in-house RV-IgG avidity assays are time-consuming and can hardly be used for single-dose testing. The method we developed for the VIDAS instrument proved to be reproducible. Dilution studies performed with RV-IgG-negative serum samples showed that RV-IgG avidity results depend, in part, on RV-IgG levels, as previously described, indicating that avidity results obtained with low-titer sera must be interpreted with caution (9).
In both techniques, 6 M urea is used as a denaturing agent, but in total, compared to the results obtained by the in-house assay, those obtained by the VIDAS instrument were lower. This observation could be due to differences between the procedures (washing protocols, secondary antibodies, etc.). With the VIDAS instrument, for naturally acquired infections, there is a clear-cut difference between the avidity indexes obtained for infections of less than 2 months and those obtained for infections of more than 3 months. Taking into account these results, an avidity index of <20% can be considered very low, one between 20% and 40% can be considered low, one between 40% and 60% can be considered moderate, and one of >60% can be considered high.
It has recently been reported that after vaccination, maturation of IgG avidity is slower than after naturally acquired infection (2, 15). The same results were obtained with the VIDAS assay. Indeed, concerning past RV-IgM-positive or -negative infections, the median VIDAS RV-IgG avidities are, respectively, 82% and 77%, whereas the median VIDAS RV-IgG avidity for past vaccinations is 58% (Fig. 2 and 3). The latter result shows that it is possible to find moderate avidity values many years after vaccination. This point has to be taken into account to confirm a diagnosis of primary infection. Besides, the possibility cannot be excluded that some high avidity values found in vaccinated people (>4 months) are due to an anamnestic response in patients with low RV-IgG levels in prevaccination serum samples because vaccination is proposed not only to people whose RV-IgG results are completely negative but also to those whose RV-IgG titers are below the cutoff of the assay. Indeed, the cutoff indicating “rubella specific immunity” has been somewhat arbitrarily set higher than the actual detection limit for specific RV-IgG.
Regarding potential interference, no false-positive results were found with serum samples with high titers of rheumatoid factor or antinuclear antibodies.
This topic has recently been addressed by G. Enders’ group, and the preliminary results reported seem to support our own conclusions (4). The RV-IgG avidity assay we developed for the VIDAS instrument provides rapid results (less than 1 h), and its automation improves the reproducibility required for an avidity test. Compared to the in-house technique, the VIDAS RV-IgG avidity assay performs well and can help confirm or exclude rubella virus infection. However, exclusion of primary infection is only possible if the first serum sample is collected early during pregnancy.
Acknowledgments
This work was supported in part by bioMérieux.
We thank Richard Keros for critical reading and English language correction of the manuscript.
Footnotes
Published ahead of print on 3 October 2007.
REFERENCES
- 1.Al-Nakib, W., J. M. Best, and J. E. Banatvala. 1975. Rubella-specific serum and nasopharyngeal immunoglobulin responses following naturally acquired and vaccine-induced infection. Prolonged persistence of virus-specific IgM. Lancet i:182-185. [DOI] [PubMed] [Google Scholar]
- 2.Best, J. M., and G. Enders. 2007. Rubella viruses: laboratory diagnosis of rubella and congenital rubella. Perspect. Med. Virol. 15:39-77. [Google Scholar]
- 3.Böttiger, B., and I. P. Jensen. 1997. Maturation of rubella IgG avidity over time after acute rubella infection. Clin. Diagn. Virol. 8:105-111. [DOI] [PubMed] [Google Scholar]
- 4.Eggers, M., M. Enders, S. Strobel, J. Piche, I. Diz, and G. Enders. 2005. A novel and rapid method for determination of rubella virus immunoglobulin G avidity, abstr. 1133_84. In 15th European Congress of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases, Paris, France.
- 5.Enders, G., and F. Knotek. 1989. Rubella IgG total antibody avidity and IgG subclass-specific antibody avidity assay and their role in the differentiation between primary rubella and rubella reinfection. Infection 17:218-226. [DOI] [PubMed] [Google Scholar]
- 6.Grangeot-Keros, L., J. C. Nicolas, F. Bricout, and J. Pillot. 1985. Rubella reinfection and the fetus. N. Engl. J. Med. 313:1547. [DOI] [PubMed] [Google Scholar]
- 7.Hedman, K., and I. Seppälä. 1988. Recent rubella virus infection indicated by a low avidity of specific IgG. J. Clin. Immunol. 8:214-221. [DOI] [PubMed] [Google Scholar]
- 8.Hedman, K., and S. A. Rousseau. 1989. Measurement of avidity of specific IgG for verification of recent primary rubella. J. Med. Virol. 27:288-292. [DOI] [PubMed] [Google Scholar]
- 9.Hedman, K., M. Lappalainen, M. Soderlund, and L. Hedman. 1993. Avidity of IgG in serodiagnosis of infectious diseases. Rev. Med. Microbiol. 4:123-129. [Google Scholar]
- 10.Macé, M., D. Cointe, C. Six, D. Levy-Bruhl, I. Parent du Châtelet, D. Ingrand, and L. Grangeot-Keros. 2004. Diagnostic value of reverse transcription-PCR of amniotic fluid for prenatal diagnosis of congenital rubella infection in pregnant women with confirmed primary rubella infection. J. Clin. Microbiol. 42:4818-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mubareka, S., H. Richards, M. Gray, and G. A. Tipples. 2007. Evaluation of commercial rubella immunoglobulin G avidity assays. J. Clin. Microbiol. 45:231-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas, H. I., P. Morgan-Capner, A. Roberts, and L. Hesketh. 1992. Persistent rubella-specific IgM reactivity in the absence of recent primary rubella and rubella reinfection. J. Med. Virol. 36:188-192. [DOI] [PubMed] [Google Scholar]
- 13.Thomas, H. I., P. Morgan-Capner, G. Enders, S. O'Shea, D. Caldicott, and J. M. Best. 1992. Persistence of specific IgM and low avidity specific IgG1 following primary rubella. J. Virol. Methods 39:149-155. [DOI] [PubMed] [Google Scholar]
- 14.Thomas, H. I., E. Barrett, L. Hesketh, A. Wynne, and P. Morgan-Capner. 1999. Simultaneous IgM reactivity by EIA against more than one virus in measles, parvovirus B19 and rubella infection. J. Clin. Virol. 14:107-118. [DOI] [PubMed] [Google Scholar]
- 15.Vauloup-Fellous, C., and L. Grangeot-Keros. 2007. Humoral immune response after primary rubella virus infection and after vaccination. Clin. Vaccine Immunol. 14:644-647. [DOI] [PMC free article] [PubMed] [Google Scholar]