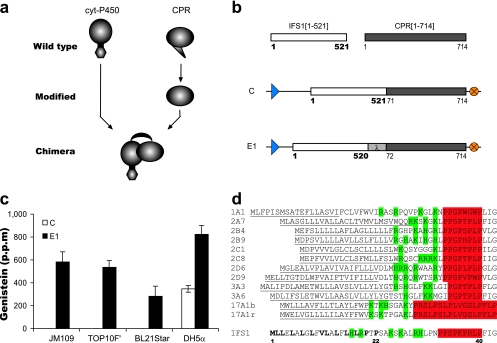
FIG. 1.
Design and functional activity of cytochrome P450 constructs. (a) General illustration of the construction of a one-component enzyme chimera from the parental cytochrome P450 (cyt-P450) and CPR proteins. (b) Construction of gene ensembles encoding a cytochrome P450, IFS1[1-521], and a CPR, CPR[1-714]. Numbers correspond to the amino acids encoded by the first and last codons of the gene sequences. All gene ensembles were flanked by a trc promoter (blue arrowhead) and an rrnB transcriptional terminator sequence (orange circle). C indicates the construct coexpressing the native full-length IFS1[1-521] and the truncated CPR[71-714] containing a start codon. The E1 one-component enzyme chimera was constructed through the formation of a translational fusion between the native IFS1[1-520] and the truncated CPR[70-714] through a linker (λ). (c) In vivo redox activity of E. coli strains expressing C and E1 upon the addition of naringenin substrate to accumulate genistein in TB medium. (d) N-terminal sequence analysis of mammalian cytochrome P450 enzymes together with IFS1 through proline-rich-region alignment. Underlined sequences represent the membrane recognition signal domain of mammalian P450 enzymes. Positively charged residues are indicated in green, and the proline-rich motif is indicated in red. For IFS1, hydrophobic amino acids ahead of the proline-rich motif are in boldface letters.