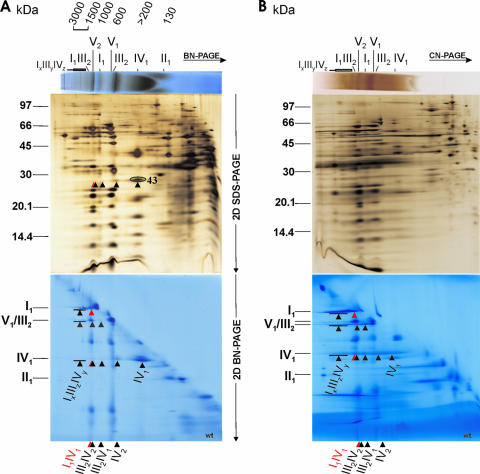
FIG. 1.
Respiratory supercomplexes and ATP synthase dimers in N. crassa wild-type mitochondria. (A and B) Digitonin-solubilized crude mitochondria were analyzed by BN-PAGE (A) and CN-PAGE (B) in the first dimension. In both panels, results are shown for in-gel activity of COX (upper panels) and subsequent 2D SDS-PAGE (silver stained) to resolve the subunits of all OXPHOS complexes and their supercomplexes (middle panels) and 2D BN-PAGE (Coomassie stained) with 0.02% DDM in the cathode buffer to dissociate the OXPHOS supercomplexes into their individual complexes (lower panels). For mass calibration, digitonin-solubilized bovine heart mitochondria were used: individual complexes I to V (130 to 1,000 kDa) and supercomplexes a to e (I1III2IV0-4; 1,500 to 2,300 kDa). Additionally, 31 subunits of all five OXPHOS complexes separated in 2D BN-SDS-PAGE (A) were verified by MALDI-MS, which are marked in detail in Fig. 2 for the sake of clarity. The OXPHOS supercomplexes were assigned according to their subunit compositions and apparent molecular masses. Besides ATP synthase monomers and dimers (V1 and V2), the individual respiratory complexes I to IV as well as the respiratory supercomplexes IxIIIyIVz, I1III2, I1IV1, III2IV2, III2IV1, and IV2 are indicated. Additionally, a subunit of complex IV (spot 43; Table 1) in the 2D SDS-PAGE (A) as well as the separated complex IV monomers, complex III dimers, and complex I monomers, which constitute the supercomplexes IV2, III2IV1, III2IV2, I1IV1, and IxIIIyIVz, in the 2D BN-PAGE (A and B) are marked by arrowheads. Note that supercomplexes I1IV1 and III2IV2 have very similar apparent masses. Similarly, the mobility of dimeric complex IV (IV2) is only slightly higher than that of complex III.