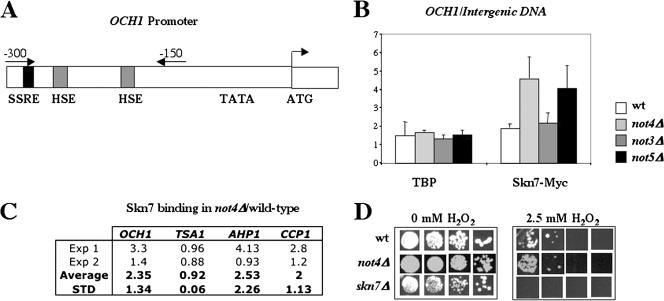
FIG. 4.
The binding of Skn7 is specifically increased on the OCH1 promoter in not4Δ cells compared to wild-type cells. (A to C) The indicated strains, wild-type (MY3683), not3Δ (MY3819), not4Δ (MY3688), and not5Δ (MY3820) expressing genomically tagged Skn7-Myc, were grown exponentially in rich medium, fixed, lysed, and incubated with anti-Myc or anti-TBP antibodies in the presence of protein G-Sepharose beads. The presence of the DNAs of interest in the immunoprecipitates was revealed by real-time PCR after purification of the DNA. (A) The positions of the oligonucleotides used for the amplification of the OCH1 promoter are depicted by arrows, and the positions of the SSRE and HSEs in the OCH1 promoter are indicated. (B) The results presented are the averages of three independent experiments and are expressed as the ratio of the presence of the OCH1 promoter (relative to the input DNA) to that of an intergenic region (relative to input DNA) in the immunoprecipitates. This value is significantly higher in the not4Δ strain than in the wild-type strain (t test, P value of 0.05). Control experiments were done to titrate the amount of Myc antibody required, and cells expressing endogenous untagged Skn7 were used as a control for specific coimmunoprecipitation of DNA by tagged Skn7. (C) The presence of several different promoters in the immunoprecipitates was assessed in two independent experiments and normalized to the intergenic DNA. Ratios of levels in the not4Δ strain to levels in the wild-type strain are presented together with the average and standard deviation (STD). (D) The indicated cells, wild-type (MY1), not4Δ (MY3595), and skn7Δ (MY3725) cells, were grown exponentially in rich medium, and then equal volumes (5 μl) of serial dilutions were spotted on yeast extract-peptone-dextrose plates containing 0 or 2.5 mM H2O2.