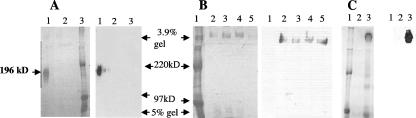
FIG. 2.
SDS-polyacrylamide gel electrophoresis of Flo11 proteins secreted from yeast cells of two strain backgrounds. In each case, the Coomassie-stained polyacrylamide discontinuous gel (3.9% stacking gel and 5% resolving gel) is shown on the left, while the corresponding Western blot using anti-six-His antibody is shown on the right. (A) Lane 1, culture supernatant of strain L5487 flo11Δ (Σ1278b background) transformed with pFLO11-GPIΔ; lane 2, culture supernatant of L5487 flo11Δ transformed with pFLO11 (GPI anchor sequences included); lane 3, protein molecular size marker. (B) Purified secreted Flo11p from the Σ1278b strain aggregated in a form that remained in the stacking gel. Secreted Flo11p from strain L5487 flo11Δ transformed with pFLO11-GPIΔ was purified on a nickel column. Lane 1, protein size marker; lane 2, fraction 7; lane 3, fraction 8; lane 4, fractions 9 and 10; lane 5, fractions 13 and 14. (C) Secreted Flo11p from S. cerevisiae var. diastaticus strain YIY345 flo-1 transformed with pFLO11-GPIΔ produced a large form that remained in the stacking gel. Lane 1, protein size marker; lane 2, culture supernatant of YIY345 transformed with pFLO11; lane 3, culture supernatant of strain YIY345 transformed with pFLO11-GPIΔ.