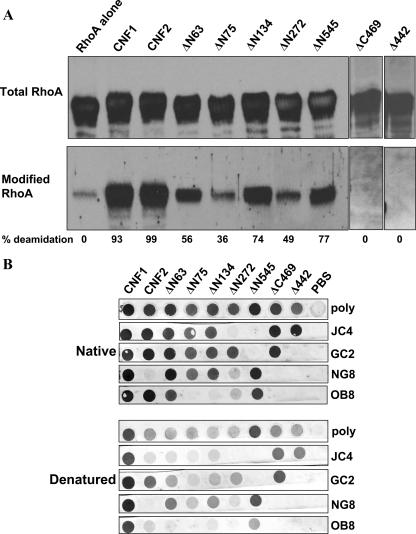
FIG. 2.
Enzymatic activity and conformation of CNF1 truncated toxins. (A) Western blot analysis of RhoA incubated in the presence or absence of purified toxins. Membranes were probed with a MAb against RhoA (top panel) or with antisera that recognize only the deamidated form of the GTPase (bottom panel), and reactive proteins were detected with appropriate HRP-conjugated secondary antibodies and the ECL substrate. The pixel density of total and modified RhoA was used to calculate the percent modification, and the results are indicated below the blots. Background values for the RhoA control were subtracted from each of the sample values. (B) Purified toxins (5 μg/well) were either applied directly to nitrocellulose membranes (upper panels) or denatured (with SDS, dithiothreitol, and boiling) prior to application (lower panels). As indicated on the right, blots were probed with either CNF1 polyclonal antisera (poly) or a panel of CNF1 MAbs, and reactive proteins were visualized with HRP-conjugated secondary antibodies and the diaminobenzamidine substrate.