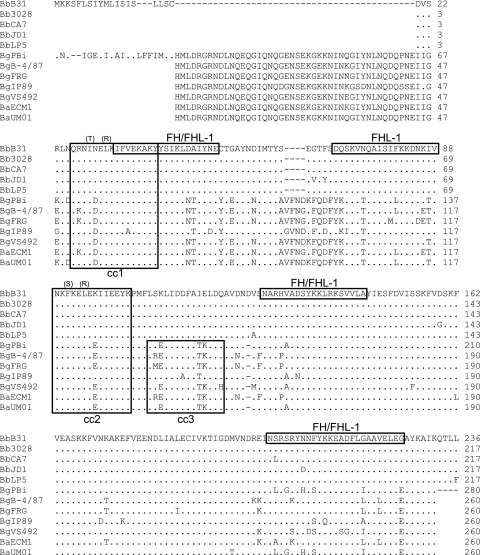
FIG. 2.
Amino acid sequence alignment of CspZ from diverse Lyme disease isolates. The nucleotide sequences of cspZ from 12 isolates (indicated to the left) were translated and aligned (with B. burgdorferi B31MI CspZ serving as the reference sequence). Blank spaces indicate regions for which sequence was not directly determined and hence do not indicate actual gaps. Gaps introduced by alignment are indicated by dashes, and residues identical to those in the reference sequence are indicated by periods. Putative FH and/or FHL-1 binding domains previously postulated for the B31MI CspZ-derived sequence are indicated by boxing of the reference sequence (9). Note that the data presented in this report do not in most cases support the designation of these linear sequence elements as serving as direct interaction sites for either FH or FHL-1. Predicted coiled-coil domains are indicated by boxing, and each is sequentially numbered (cc1, cc2, or cc3). Amino acid substitutions introduced into B. burgdorferi B31MI CspZ through site-directed mutagenesis are indicated in parentheses above the targeted residues. Abbreviations: Bb, B. burgdorferi; Bg, B. garinii; Ba, B. afzelii.