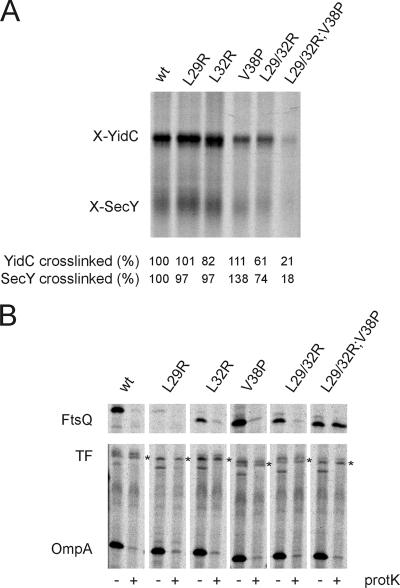
FIG. 3.
Targeting of the FtsQ mutants to SecY/YidC and translocation of the mutants. (A) In vitro cross-linking of FtsQ nascent chains to YidC and SecY. In vitro translation of nascent 108FtsQ was performed in an E. coli cell- and membrane-free extract in the presence of [35S]methionine. After addition of IMVs, targeting of ribosome-nascent complexes to the membrane was assayed using a photoactivatable cross-linker incorporated into the FtsQ TMS. Inner membrane proteins were separated from soluble and membrane-associated proteins by carbonate extraction, and the inner membrane protein fraction was analyzed for cross-linked adducts to 108FtsQ. Cross-linked adducts to SecY and YidC were identified, and the ratio of radiolabeled cross-linked adduct to the total amount of labeled nascent chains present in the reaction mixture was determined. Subsequently, cross-linking efficiency was calculated from these ratios, with cross-linking to 108FtsQ set at 100%. (B) Proteinase mapping of FtsQ translocation. FtsQ and the FtsQ mutants were expressed and pulse-labeled in Top10F cells. The cells were converted to spheroplasts and treated with proteinase K to monitor translocation of FtsQ across the cytoplasmic membrane. The samples were immunoprecipitated using antibodies directed against FtsQ (top panel) or antibodies directed against OmpA and TF (bottom panel). OmpA (outer membrane protein A) and TF (cytoplasmic protein; asterisk) were analyzed as controls for proteinase K treatment and spheroplast formation, respectively. Immunoprecipitated material was analyzed by SDS-PAGE and visualized by phosphorimaging. The proteinase-sensitive band below TF is an unidentified protein that is routinely found in TF/OmpA immunoprecipitates (38). wt, wild type.