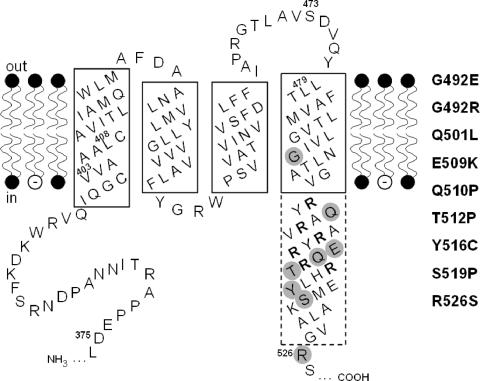
FIG. 1.
Secondary structure model of the transmembrane region of KdpD. The hydrophobic membrane-spanning domains in the phospholipid bilayer as well as parts of the adjacent hydrophilic domains are depicted. Amino acid numbers refer to positions in the KdpD protein. α-Helical regions believed to be embedded in the membrane are framed by a solid line, while the part of the fourth helix that extends into the cytoplasm is drawn with a dashed-line boundary. The arginine residues of the arginine cluster are shown in bold letters, and the three regions (I, II, and III) corresponding to DNA sequences that were subjected to random mutagenesis have been indicated by their first and last amino acid (for details, see Materials and Methods). The altered amino acids leading to a K+-insensitive phenotype are shaded gray, and the corresponding amino acid replacements are presented on the right hand.