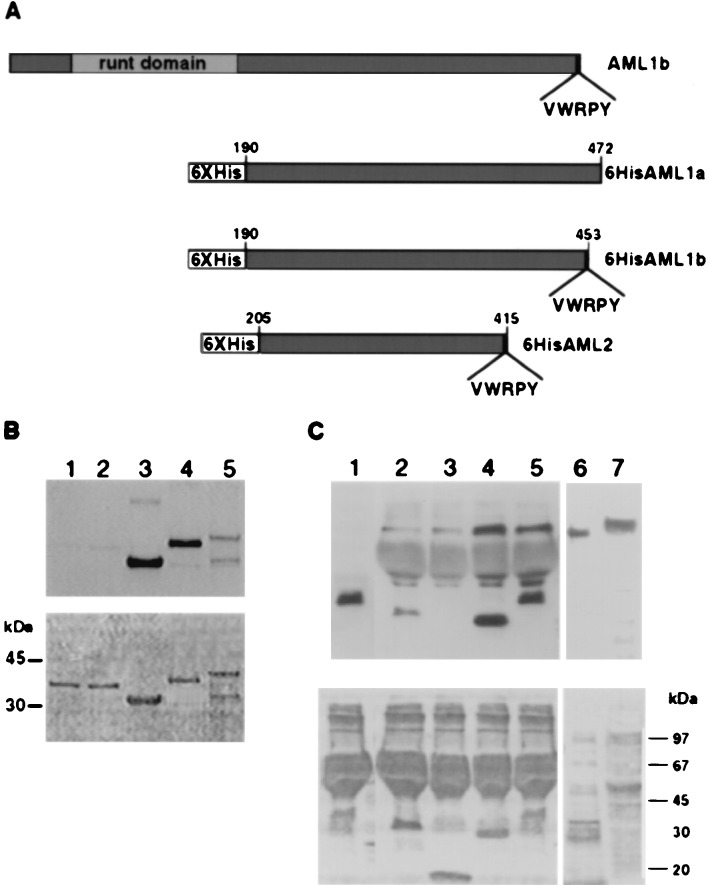
Figure 2.
VWRPY-containing AML proteins associate with Gro and TLE. (A) A scheme of 6× His AML proteins used in Far Western and pull-down analyses. Numbers on the 6× His derivatives indicate the regions that are included in the constructs. (B) In vitro association assay between AML and TLE proteins. Three micrograms of 6× His proteins: AML2, 33 kDa, lane 3; AML1b, 37 kDa, lane 4; AML1a, 39 kDa, lane 5; and control nonrelevant fused proteins, Bovine α1 and α2 PAF-AH (1b) subunits of 35 kDa, lanes 1 and 2, were analyzed by Western blotting and reacted with 35S in vitro -translated TLE1. (C) Interaction in Jurkat cell extracts between immobilized AML and TLE. 6× His-AML1-b lane 5, AML2 lane 4 and control nonrelevant proteins: 6× His leptin lane 3 (18 kDa) and the α2 PAF-AH (1b) subunit lane 2 (35 kDa). Bound proteins were analyzed with pan-TLE monoclonal antibodies. Lane 1 AML1-b without Jurkat extract, served as control for nonspecific binding of anti-TLE antibodies to 6× His proteins. The size difference between in vitro-translated TLE (lane 6) and Jurkat extract TLE (lane 7) may result from expression of four different TLE genes in Jurkat cells. Ponceau staining are shown in the bottom panels of B and C.