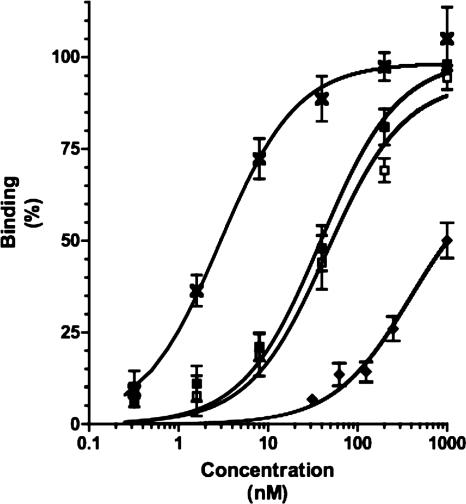
FIG. 6.
Binding of unglycosylated mature AIDA-I to cultured epithelial cells. The glycosylated (open squares) or unglycosylated (closed squares) fusion of GST to mature AIDA-I (encompassing amino acids 50 to 847) was purified by affinity chromatography. Various amounts of the proteins were incubated with fixed Hep-2 cells. Pure GST (diamonds) and pure whole AIDA-I (crosses) were used as controls. Bound proteins were revealed with an anti-GST antiserum directly coupled to HRP or an anti-AIDA-I serum and a secondary antibody coupled to HRP. Background values were subtracted, and the absorption was normalized by dividing the absorption of each well by the maximal absorption measured on the plate in order to obtain percentages of maximal binding.