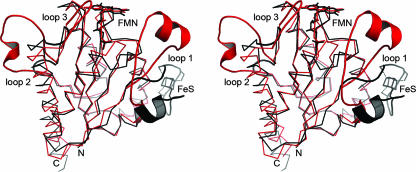
FIG. 6.
Superposition of the monomers of E. coli WrbA (red) and M. thermophila ISF (black). Based on a common flavodoxin-like fold, the structures differ in three characteristic loop regions. Strongest variations are visible in the loop region 1, where ISF binds a [4Fe:4S] cluster while WrbA forms a specific binding cleft for NADH at a very different position in the monomer. However, multimer formation then places both putative electron donor sites at very similar positions in respect to the FMN cofactor.