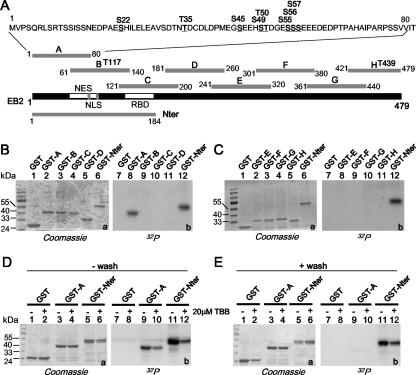
FIG. 4.
CK2 in HEK293T cell extracts binds to and phosphorylates the EB2 N terminus. (A) Schematic representation of the EB2 peptides fused to GST to generate GST-A, -B, -C, -D, -E, -F, -G, -H, and -Nter. Putative CK2 sites shown over the sequences of peptides A, B, and H were determined using Scansite (http://scansite.mit.edu). (B and C) Glutathione-Sepharose 4B beads loaded with GST and the GST fusion proteins indicated above the lanes were incubated for 30 min at 30°C with a HEK293T cell extract supplemented with kinase buffer and [γ-32P]ATP. GST and the GST fusion proteins were eluted, resolved by SDS-PAGE, and visualized by Coomassie blue staining (gels a) and by autoradiography (gels b). (D and E) Glutathione-Sepharose 4B beads loaded with GST and the GST fusion proteins indicated above the lanes were incubated overnight with a HEK293T cell extract. The − wash assays were supplemented with kinase buffer and [γ-32P]ATP and further incubated for 30 min at 30°C. For the + wash assays, the beads were washed extensively with MTPBS and then submitted to a kinase assay. Where indicated, 20 μM TBB was added to the assay. GST and GST fusion proteins were eluted, resolved by SDS-PAGE, and visualized by Coomassie blue staining (gels a, lanes 1 to 6) and by autoradiography (gels b, lanes 7 to 12).