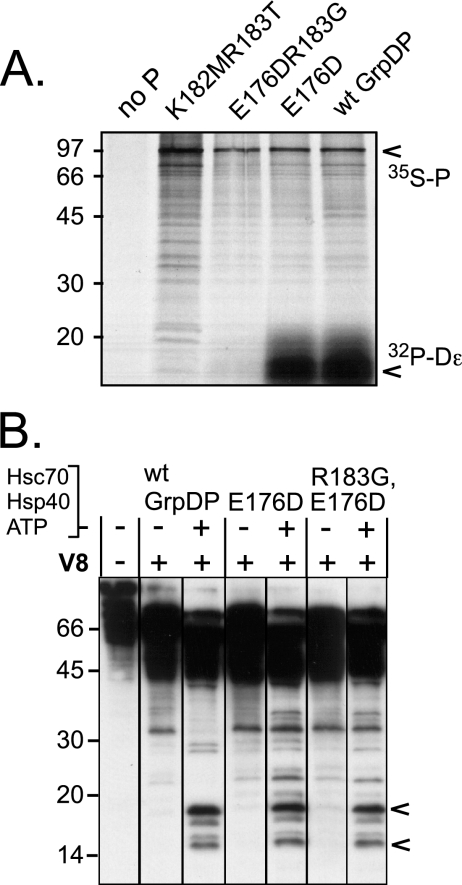
FIG. 8.
RNA-binding deficiency of P mutant R183G is not caused by a gross structural alteration. (A) The single R183G mutation ablates Dɛ RNA binding as efficiently as a K182MR183T double mutation. The indicated proteins were in vitro translated in RL in the presence of [35S]methionine and 32P-labeled Dɛ RNA and immobilized via their His tags to Ni-NTA agarose. Protein and potentially bound RNA were analyzed by SDS-PAGE and autoradiography. The positions of 35S-labeled P protein and of 32P-labeled RNA are indicated by arrowheads. (B) The RNA-binding-deficient R183G mutant undergoes the same chaperone-induced conformational change as RNA-binding-competent P protein. The indicated proteins were incubated with Hsc70, Hsp40, and ATP, or not and subjected to limited V8 proteolysis, followed by immunoblotting with MAb9. All samples were run on the same gel, except that the lanes have been reordered for ease of comparison between chaperone-treated and nontreated samples from one protein. The R183G mutant produced essentially the same chaperone-induced fragments as wild-type GrpDP and the parental, RNA-binding-competent variant E176D. wt, wild type.