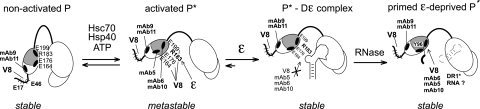
FIG. 9.
Structural model for hepadnaviral P protein activation. Initially, P protein is in a stable nonactivated state, in which the C-proximal TP region including E164, E176, and E199 and the epitopes for MAb5, MAb6, and MAb10 is occluded, whereas the N-proximal E residues in TP and the epitopes for MAb9 and MAb11 are, and remain, accessible. Occlusion might involve the RH domain, as suggested by the partial chaperone independence of P proteins with large C-terminal truncations (2, 43). ATP-consuming Hsc70 plus Hsp40 action transiently exposes the C-proximal sites, including R183, which is involved in RNA binding. In this metastable activated state P*, the corresponding MAbs, and ɛ can compete for binding to the C-proximal TP region. ɛ binding generates a stable priming-competent P*-Dɛ complex in which the RNA blocks the access of V8 protease and the respective MAbs. RNase A treatment creates a new state P′ in which the same sites are exposed as in chaperone-activated P*; however, different from P*, P′ remains stable without ATP-consuming chaperone activity. P′, here artificially created by RNA digestion, may resemble P protein in transition from ɛ-dependent initiation to elongation mode, which likely accompanies the replacement of ɛ by DR1* as the RNA template.