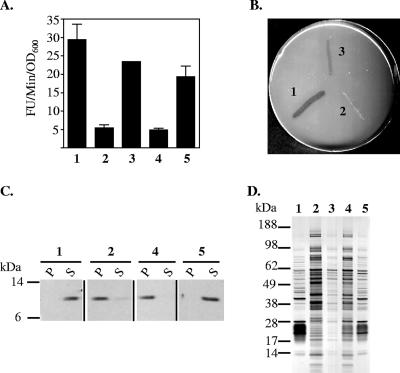
FIG. 2.
Inverse correlation between T2S and passive leakage. Wild-type and eps mutant strains of V. cholerae TRH7000 were analyzed for the secretion of protease, lipase, and toxin, as well as for the release of cellular proteins to the extracellular environment. (A) Culture supernatants were obtained from stationary-phase cultures as described in Materials and Methods and were tested for the presence of extracellular protease using the proteolytic substrate N-tert-butoxy-carbonyl-Gln-Ala-Arg-7-amido-4-methyl-coumarin. The rate of hydrolysis (relative fluorescence units [FU]/min/OD600 unit) is presented as the means of three independent experiments ± SEM. There was a statistically significant difference between the protease activity of wild-type and eps mutant strains (P < 0.005). (B) Lipase secretion was determined by growth on lipid agar containing olive oil as the only source of carbon. (C) The distribution of the E. coli heat-labile enterotoxin B subunit in culture supernatants (S) and periplasmic extracts (P) was determined by SDS-PAGE and immunoblotting using monoclonal anti-EtxB. (D) Culture supernatants were obtained from stationary-phase cultures, matched by equivalent OD600 units, and analyzed by SDS-PAGE and silver staining for total protein content. Lane 1, wild type; lane 2, TRHΔeps; lane 3, TRHΔeps+pEps; lane 4, PBAD::eps; lane 5, PBAD::eps grown in the presence of arabinose at a 0.01% final concentration.