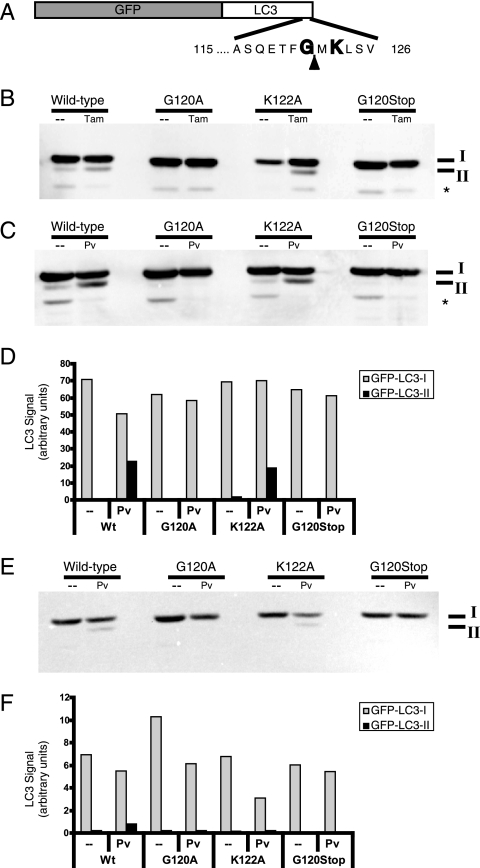
FIG. 2.
Effect of mutations in GFP-LC3 on modification during autophagy induction and poliovirus infection. (A) Schematic depiction of the GFP-LC3 fusion protein sequence. The solid arrowhead indicates the site of cleavage by cellular protease Atg4. Gly120 is the known site of phosphatidylethanolamine addition during autophagy induction. (B) DNA plasmids that encode wild-type GFP-LC3 and variant GFP-LC3 sequences containing G120A, G120Stop, and K122A mutations were transfected into 293T cells. Following transfection, cells were fed with either normal medium (-) or medium that contained 10 μM tamoxifen for 48 h. Cell extracts were displayed on a 10% polyacrylamide-SDS gel and immunoblotted using an anti-LC3 antibody. The asterisk signifies an unidentified cross-reacting band. (C) Wild-type and G120A, K122A, and G120Stop mutant GFP-LC3-encoding plasmids were transfected into 293T cells. After 48 h, cells were infected with poliovirus (50 PFU/cell) and harvested after 6 h. GFP-LC3 protein was detected as for panel B. Gels are representative of multiple experiments. (D) Quantitation of data in panel C is shown. The anti-LC3 immunoblot in panel C was reprobed with an antibody to GFP (E) to test whether the relative immunogenicity of GFP-LC3-I and GFP-LC3-II changed with different antibodies. (F) Relative amounts of GFP-LC3-I and GFP-LC3-II are quantified.