Abstract
The activation of dendritic cells is marked by changes both on their cell surfaces and in their functions. We define EWI-2/CD316 as an early activation marker of dendritic cells upregulated by Toll-like receptor ligands clearly before CD86 and CD83. By expression cloning, human heat shock protein A8 (HSPA8), a member of the hsp70 family, was identified as the ligand for EWI-2. Soluble EWI-2 bound both to cells expressing HSPA8 and also to immobilized HSPA8 protein. Although heat shock proteins are evolutionarily well conserved, other members of this class, including human hsp60 and mycobacterial hsp65, did not bind to EWI-2. The ligation of EWI-2 enhanced the CCL21/SLC-dependent migration of activated mature dendritic cells but attenuated their antigen-specific stimulatory capacities. Important functions of recently activated dendritic cells are thus critically modulated by the newly discovered HSPA8-EWI-2 interaction.
Dendritic cells are the most crucial sentinels of the immune system. Although different DC sublineages have heterogeneous phenotypes and functions, they share similarities in their maturation processes (2, 3). First, precursors are generated continuously in the bone marrow. They circulate as immature DCs and then enter their destination compartment, where they mature. These resident DCs then are activated by danger signals derived primarily from pathogens and possibly also from endogenous metabolic processes. The activation step is very rapid and is characterized by cellular and morphological changes: the upregulation of surface markers, the expression of cytokines and chemokines, and the formation of dendrites. Several such activation/maturation markers have been described, including, for example, members of the B7 superfamily, like CD80 and CD86, which then later provide costimulatory signals during the priming of effector cells.
However, the appearance of these already known molecules follows the activation event with a certain delay. We wanted to understand the very early changes on the dendritic cell surface after stimulation, because we reasoned that surface molecules appearing immediately early after stimulation might be involved in controlling the maturation and fate of the DC itself. For example, immediate early activation molecules could amplify the external activation signal, could modify the migratory behavior, or could serve as a stop signal by initiating a negative feedback loop. We searched for such first-line activation markers by comparing naïve and early activated DCs in a differential display analysis. Here we identify EWI-2 as an early induced transcript whose presence on dendritic cells has not been described before. EWI-2 (11) is also known as CD316, PGRL (PG regulatory-like protein), KAI/CD82-associated surface molecule (34), and CD81-binding partner 3 (CD81P3) (10). EWI-2 contains four immunoglobulin domains, a transmembrane region, and a short cytoplasmic tail that does not bear any signature motif for signal transduction. However, work of the Hemler and Rubinstein groups indicated that EWI-2 participates in a multitude of interactions and is also part of the tetraspanin web and that EWI-2 regulates integrin α3β1 as well as α4β1 functions (11, 17, 24).
Our efforts to find a ligand for EWI-2 whose binding would initiate signaling with maturation-modifying properties identified HSPA8, a member of the heat shock protein family, as an EWI-2 ligand. Heat shock proteins are highly conserved and ubiquitously expressed proteins whose expression levels and availabilities are actively regulated in infections and other stressful conditions. Several families of HSPs exist, including the HSP70 subfamily. To date, 12 members of the human HSP70 subfamily have been isolated. The constitutive isoform HSPA8 is also termed HSP73 and HSC70/HSC73. It is expressed in the cytosol, where HSP73 functions as a molecular chaperone for newly synthesized proteins. However, HSPA8 can also be found extracellularly in a membrane-bound form, for example, on the surfaces of Epstein-Barr virus-transformed B cells and cancer cells (14). HSPA8 was also reported to be a putative cell surface marker for undifferentiated human embryonic stem cells (22). Barreto and colleagues (4) showed the release of HSC70, corresponding to HSPA8, from human tumor cells upon stress. High levels were detected following heat shock or bacterial infection. HSPA8 can also be found at the surfaces of tumor T cells and epithelial cancer cells and also as a soluble molecule in the synovial fluid of rheumatoid arthritis patients (21). The functions of these membrane-bound and secreted hsp forms are still mostly unknown.
We also show that the interaction between EWI-2 and HSPA8 has functional consequences on the behavior of activated dendritic cells. In fact, these two molecules may be members of a broader regulatory circle that fine-tunes the transition between innate and adaptive immune responses.
MATERIALS AND METHODS
Terms and abbreviations used.
Terms and abbreviations used in this work are as follows: BSA, bovine serum albumin; DC, dendritic cell; EF, elongation factor; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; FCS, fetal calf serum; FITC, fluorescein isothiocyanate; GM-CSF, granulocyte-macrophage colony-stimulating factor; HKLM, heat-killed Listeria monocytogenes; HSP, heat shock protein; h, human; IFN-γ; gamma interferon; Ig, immunoglobulin; IL, interleukin; IPTG, isopropyl-β-d-thiogalactopyranoside; LC, Langerhans cell; LPS, lipopolysaccharide; LTA, lipoteichoic acid; MAb, monoclonal antibody; MALP-2, macrophage-activating lipopeptide from Mycoplasma fermentans; MoDC, monocyte-derived dendritic cell; PAMP, pathogen-associated molecular pattern; PBMC, peripheral blood mononuclear cell; PBS, phosphate-buffered saline; PE, phycoerythrin; rh, rhesus; RT-PCR, reverse transcription-PCR; sEWI-2, soluble EWI-2; siRNA, small interfering RNA; TCC, T-cell clones; TGF-β, transforming growth factor β; TLR, Toll-like receptor; TNF-α, tumor necrosis factor alpha.
Antibodies and reagents.
Monoclonal anti-EWI-2 antibodies were generated by immunization of BALB/c and ABH/Biozzi mice with irradiated EWI-2 transfectants and/or sEWI-2 protein. Spleen cells were fused with SP2/0 mouse myeloma cells by use of standard techniques. The following antibodies (MAb clones) were used in this study: anti-CD86 PE (BU63, IgG1) (Cymbus Biotechnology, NH); IgG1 PE (G18-145), anti-CD11c (B-ly6, IgG1), and IgG1-biotin (MOPC-31C) (BD, NJ); anti-TLR2 (TL2.1, IgG2a) and anti-TLR4 (HTA125, IgG2a) (eBioscience, CA); IgG2a (MCA929XZ) (Serotec, Germany); anti-CD123/IL-3Rα PE (AC145, IgG2a) (Miltenyi Biotec, Bergisch Gladbach, Germany); hIgG sera (Sigma, MO); and mouse IgG sera (Pierce). Second-step reagents were anti-mouse PE (Biosource, CA) and streptavidin-allophycocyanin (BD, NJ). Recombinant human cytokines used were as follows: rhGM-CSF, rhIL-4, and rhIL-3 (Novartis AG, Basel, Switzerland); rhSCF (R&D Systems, Minneapolis MN); rhTNF-α (30 ng/ml) (Sigma, MO); IL-1β; FLT3L, and TGF-β (R&D Systems, Minneapolis MN); and IFN-γ (Strathmann Biotec, Germany). Reagents that were used for stimulation were as follows: LPS, LTA, poly(I:C), zymosan, peptidoglycan, TNF-α, and ionomycin (Sigma, MO); CpG (TIB Molbio, Germany); flagellin (Alexis, Oxford, United Kingdom), and fibronectin (BD, CA); HKLM, MALP-2, and Pam3CSK4 (InvivoGen, CA); and imiquimod (SeqChem, United Kingdom).
Purification and preparation of DC subsets.
Lymphocyte-enriched peripheral blood concentrates derived from healthy donors were obtained from the Vienna General Hospital. PBMCs were isolated by centrifugation over a Ficoll-Paque Plus (Amersham Bioscience, Uppsala, Sweden) gradient. Monocytes were purified from PBMCs by negative sorting using a monocyte isolation kit (all magnetic isolation kits from Miltenyi Biotec, Bergisch Gladbach, Germany). MoDCs were generated by culturing monocytes in RPMI complete medium supplemented with 100 ng/ml rhGM-CSF and 80 ng/ml rhIL-4 for 5 days at 0.5 × 106 cells/ml. Myeloid DCs were generated from PBMCs by use of a BDCA-1 positive isolation kit. Myeloid DCs were stimulated directly after isolation. Plasmacytoid DCs were obtained from PBMCs by positive sorting using anti-BDCA-2-conjugated magnetic microbeads. Plasmacytoid DCs were cultured and activated in RPMI complete medium supplemented with 100 ng/ml rhIL-3. LCs were generated from umbilical cord blood samples according to the method of Caux et al. (9) as follows. CD34+ cells were isolated from mononuclear fractions by positive selection using anti-CD34-conjugated magnetic microbeads. LC cultures were established in RPMI complete medium supplemented with 50 ng/ml GM-CSF, 50 ng/ml FLT3L, and 20 ng/ml SCF. Cells were then washed on day 2 and cultured in RPMI complete medium containing 50 ng/ml GM-CSF, 100U/ml TNF-α, and 5 ng/ml TGF-β for 3 days.
Migration assay.
DC migration was evaluated using a 24-well, 5.0-μm-pore-size Transwell plate (Costar, Cambridge, MA). DCs were washed once with PBS and adjusted to 2 × 106 cells/ml in assay medium (RPMI 1640 without phenol red, 0.1% BSA). An aliquot (50 μl) of this cell suspension was placed in the top Transwell chamber. Optimal concentrations of chemokines were determined first in preexperiments (500 ng/ml of SLC/CCL21, 200 ng/ml of MIP-3β/CCL19, 100 ng/ml SDF-1α/CXCL12, or 100 ng/ml SDF-1β/CXCL12; R&D Systems, Minneapolis MN). Optimal chemokine concentrations were then added to the bottom chamber. Antibodies were added to the top as well as the bottom chamber. After 180 min of incubation at 37°C in a 5% CO2 atmosphere, the top chamber was removed, and cells that migrated into the bottom chamber were counted.
Cell culture and cell lines.
The following cell lines were purchased from the American Type Culture Collection (Manassas, VA): CEM (CCL-119), HeLa (CCL-2), HuT78 (TIB-161), U266 (TIB-196), U937 (CRL-1593), and PA-1 (CRL-1572). PEER (ACC6) and SP2/0 (ACC125) were from DSMZ (Braunschweig, Germany). Adherent cells were cultured in Dulbecco minimal essential H21 medium, and cells in suspension were cultured in RPMI 1640 complete medium containing 10% FCS, 1% penicillin, and 1% streptomycin (InvivoGen, CA) at 37°C and 5% CO2.
The CD4+ human TCC, referred to as CFTS4:3.1, CFTS4:2.15, and CFTS4:2.20, were established from punch biopsy samples of clinically noninvolved skin from an atopic dermatitis patient, taken 24 h after epicutaneous challenge with a protein extract of house dust mites as previously described (31). The TCC recognize peptides of major allergen Der p1 of Dermatophagoides pteronyssinus in association with the major histocompatibility complex class II restriction molecule HLA-DPw4 (allele HLA-DPB1*0401) (5). For expansion, the T cells were activated every 14 days via anti-CD3 MAb (MAb clone HIT3a; Pharmingen, San José, CA) immobilized via plastic-surface-bound goat anti-mouse IgG-specific antibodies and cultured in RMPI 1640 medium supplemented with 5% FCS, 5% human serum (PAA Laboratories, Linz, Austria), rhIL-2, and rhIL-4 (50 U/ml each).
For antigen-specific stimulation, DCs were incubated overnight in culture medium containing 50 μg/ml Dermatophagoides pteronyssinus protein extract (Dpt; ARTU Biologicals NV, Lelystad, The Netherlands). The content of major allergen Der p1 in the lot used was 18.5 μg/mg extract. DCs incubated without antigen served as negative controls. Following washing to remove excess antigen, DCs and 105 Der p1-specific T cells of clone 4.3.1 were mixed and seeded into 96-well culture plates at a DC/T-cell ratio of 1/40.
Generation and screening of cDNA expression library.
A Jurkat cDNA library was generated using the SMART approach (Clontech) with the modifications of Wellenreuther et al. (33) Briefly, poly(A)+ RNA was isolated (QIAGEN) and first-strand cDNA was synthesized using a Creator SMART cDNA library construction kit (Clontech, CA). Double-stranded cDNA synthesis was performed by a primer extension method, and the resulting cDNA was size fractionated on a 0.8% agarose gel. Four pools of cDNA were extracted and separately PCR amplified. The amplified cDNA was SfiI digested for 1 h at 50°C and ligated into a modified pCX4 retroviral vector (kind gift of Tsuyoshi Akagi, Osaka Bioscience Institute [1]). Each sublibrary comprised more than 250,000 primary clones. The libraries were introduced by retroviral transfection in the mouse SP2/0 cell line. EWI-2 binding cells were enriched with repeated rounds of magnetic and FACS steps with sEWI-2-hIg protein in combination with mouse anti-hIg microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and anti-hFc FITC reagent (Sigma, MO), respectively. hIgG served as the negative control (Sigma, MO). Retroviral inserts were retrieved by a two-step nested PCR amplification with vector-specific primers from genomic DNA isolated from single-cell clones.
Expression and purification of recombinant proteins.
The sEWI-2-hIg protein comprised four extracellular domains, including amino acids 1 to 574 fused to the J-CH2-CH3 domains of the hIgG heavy chain. The sEWI-2-hIg protein was expressed in Sf9 insect cells and purified from the supernatant on either recombinant protein A (Amersham Biosciences, Uppsala, Sweden)- or anti-hFc-specific affinity columns (Sigma, MO). Protein concentration was determined in an anti-hIg ELISA. For the generation of the HSPA8-His protein, the HSPA8 cDNA retrieved from the Jurkat expression library was C-terminally tagged and expressed in BL21(LysS) cells. Expression was induced with 0.5 mM IPTG for 4 h at 28°C, and the HSPA8-His protein was extracted in a buffer containing 10 mM imidazole, 10% glycerol, and 0.5% Triton X-100. HSPA8-His protein was purified on Ni-nitrilotriacetic acid agarose (QIAGEN), and residual endotoxin was removed on a polymyxin B column (Sigma, Missouri). HSPA8 was quantified using Bradford analysis, and LPS content was measured by Limulus amebocyte lysate assay. The purities and identities of both proteins were determined by use of sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblots that were developed with specific antibodies.
Flow cytometric analysis.
Cells were prepared in FACS buffer (PBS containing 0.05% sodium azide, 1 mM EDTA, and 2% FCS) and labeled with a combination of antibodies and their appropriate isotype-matched control antibodies. The binding of unlabeled antibodies was detected by fluorochrome-labeled second-step reagents. Samples were analyzed using a FACSCalibur flow cytometer (BDIS) with CellQuest acquisition software (BDIS) or the WinMDI2.8 program. The percentage of positive cells was calculated as the percentage of cells stained with specific antibodies compared to background staining, which was evaluated with corresponding isotype controls.
siRNA treatment.
HSPA8-specific siRNA duplexes were from the HiPerformance series from QIAGEN (Hildesheim, Germany) with the target sequences AAGGACCTAAATTCGTAGCAA (Hs_HSPA8_6_HP) and AACACTGTATTGTAAGTGGAA (Hs_HSPA8_8_HP). AllStars negative control siRNA was from the same supplier. Jurkat cells in the log growth phase were harvested and resuspended at 8 × 106 cells/ml in 200 μl Cytomix buffer (30) containing annealed siRNA at a 200 nM final concentration. Within 10 min, samples were transferred to a 2-mm electroporation cuvette (Bio-Rad) and treated with a single pulse of 175 V and 1,000 μF by use of a Bio-Rad electroporation apparatus. Cells were rested in 12-well plates in complete medium for 1 h and then washed with PBS and incubated in PBS containing 2% BSA and 2 mM EDTA for 15 min at room temperature. Cells were then washed again in this medium and subsequently transferred into fresh wells in complete culture medium and incubated until their analysis.
Quantitative RT-PCR.
Total RNA was isolated from cells by use of an Absolut RNA RT-PCR miniprep kit (Stratagene, CA). RT-PCR was performed with 700 ng of total RNA by use of TaqMan reverse transcription reagents (Applied Biosystems, CA) and a TaqMan Universal PCR master mix, NoAmpErase (Applied Biosystems, CA). The following primers and their corresponding probes were purchased from Applied Biosystems: CD86 forward, 5′-TTC TGA ATG AGG TAT ACT TAG GCA AAG AG-3′; CD86 reverse, 5′-GCC CTT GTC CTT GAT CTG AAG A-3′; CD86 probe, 5′-CGG ACA GTT GGA CCC TGA GAC TTC ACA-3′; EF-1α forward, 5′-TTT GAG ACC AGC AAG TAC TAT GTG ACT-3′; EF-1α reverse, 5′-TCA GCC TGA GAT GTC CCT GTA A-3′; EF-1α probe, 5′-TCA TTG ATG CCC CAG GAC ACA GAG AC-3′; and EWI-2 primer and probe set Hs00364603_m1 (Applied Biosystems).
RESULTS
Rapid activation-dependent expression of CD316/EWI-2 on DCs.
To obtain a better understanding about the repertoire of human DC molecules that are upregulated upon maturation, a representational differential analysis was undertaken. One of the molecules clearly upregulated in this assay was provisionally named GID2 (for gene induced in DCs) (data not shown). GID2 turned out to be a member of the Ig superfamily that was designated very recently as CD316 (32). The more widespread name is EWI-2, since it belongs to a distinct group that contains a characteristic glutamic acid-tryptophan-isoleucine 3-amino-acid motif.
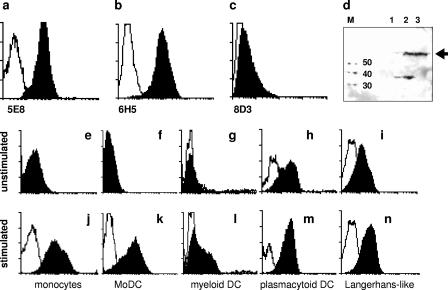
As reagents were not readily available, MAbs were raised in order to investigate the expression, localization, and function of EWI-2. Three antibodies were selected which showed specific binding in ELISA using purified protein and in FACS analysis using BaF/3 transfectants. Among them, clone 5E8 also recognized the EWI-2 protein in Western blotting (Fig. 1a to d). Although EWI-2 was not previously described to be expressed on DCs, our differential display data predicted that the protein will appear on the surfaces of DCs upon activation. Therefore, various DC subsets were isolated and tested before and after LPS-plus-IFN-γ treatment. Confirming the prediction, EWI-2 was absent on unstimulated monocytes, MoDCs, and myeloid DCs, but each of these cell types substantially upregulated EWI-2 expression upon stimulation (Fig. 1j to l). On plasmacytoid DCs and on cord blood-derived Langerhans-like cells, EWI-2 was constitutively expressed (Fig. 1 h and i).
FIG. 1.
Induction of CD316/EWI-2 on DCs. (a to d) Three different novel anti-EWI-2 mAbs were characterized by FACS staining of BaF/3 transfectants expressing full-length EWI-2: 5E8 (a), 6H5 (b), and 8D3 (c) (filled profiles; empty profiles are the isotype controls). (d) On Western blots, clone 5E8 specifically recognizes EWI-2 from PEER cells (lane 3) and from transfected SP2/0 cells (lane 2), whereas parental SP2/0 cells are negative (lane 1). The expected size (molecular mass [M]) of EWI-2 is ∼65 kDa, as indicated by the black arrow. (e to n) Expression of EWI-2 was analyzed by FACS on monocytes (e and j), MoDCs (f and k), myeloid DCs (g and l), plasmacytoid DCs (h and m), and in vitro generated Langerhans-like cells (i and n) before (upper row) and after (lower row) stimulation. Filled profiles, mAb 5E8; empty profiles, isotype controls.
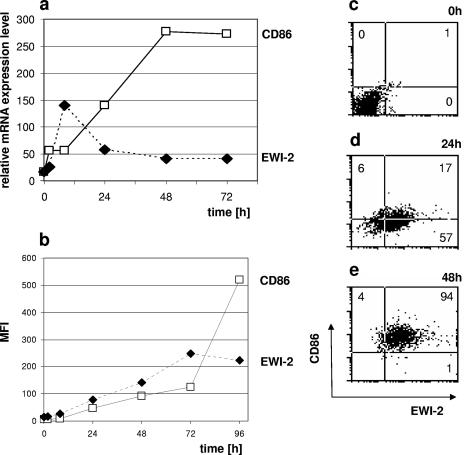
A time course analysis using real-time RT-PCR revealed that the message for EWI-2 is induced surprisingly early, with a peak already being reached after 12 h (Fig. 2a). This is clearly earlier than the upregulation of other established DC activation markers, such as CD86, which requires 2 days to reach its peak expression. EWI-2 also precedes CD86 at the protein level, but because of the lower maximum levels of surface EWI-2, the difference in the kinetics is less apparent (Fig. 2b). However, upon direct comparison, it is clear that resting DCs (Fig. 2c) first display EWI-2 upon activation (Fig. 2d), while the expression of CD86 follows only later (Fig. 2e).
FIG. 2.
Expression kinetics of EWI-2 on DCs. (a) Expression of EWI-2 and CD86 was analyzed on MoDCs after LPS-plus-IFN-γ stimulation (time = 0 h) by real-time quantitative RT-PCR at different time points. The expression level relative to EF-1α (EF-1α expression level, 1,000) is given. (b) In parallel, the surface expression levels of both molecules were measured by FACS (mean fluorescence intensity [MFI]). (c to e) Changes in cell surface EWI-2 and CD28 were directly compared by two-color FACS at three time points.
Selected TLRs and their ligands can induce EWI-2 expression.
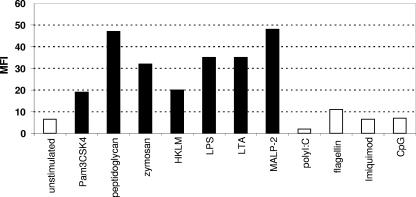
We then wanted to understand the pathways and receptors that could explain this unexpectedly rapid induction of EWI-2 expression. TLRs were among the possible candidates. Quantitative RT-PCR showed that TLR1, TLR2, and TLR4 were highly expressed on MoDCs, whereas all other TLRs were expressed at very low levels, with TLR9 not being detectable at all (data not shown). Various stimulants of TLRs were then added to the DCs, and the expression of EWI-2 was detected by FACS analysis. Consistently with the receptor expression pattern, agents acting through TLR2 and TLR4, such as Pam3CSK4, peptidoglycan, zymosan, HKLM, LPS, LTA, and MALP-2, all upregulated EWI-2 on MoDCs (Fig. 3). Flagellin, which binds to TLR5, imiquimod (TLR7/8), and poly(I:C) (TLR3), did not induce EWI-2 expression. As MoDCs and myeloid DCs do not express TLR9, they also failed to induce EWI-2 expression in response to CpG (Fig. 3). These data indicate that at least two different TLR can mediate the upregulation of EWI-2.
FIG. 3.
TLR-dependent upregulation of EWI-2. MoDCs were incubated with various TLR ligands at optimal concentrations. Expression of EWI-2 was measured by FACS (mean fluorescence intensity [MFI] is given). Black bars indicate ligands which caused significant upregulation.
HSPA8 is the ligand of EWI-2/CD316.
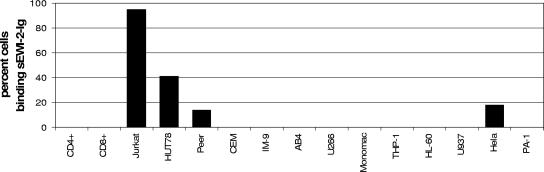
The structure of EWI-2 inferred that it could serve as a cell-cell interaction molecule or be part of a receptor/ligand system. However, no ligand or binding partner for EWI-2 was known. We thus assumed that this unknown ligand would be (i) a secreted, soluble, or cell surface-anchored molecule and therefore accessible for FACS detection and (ii) genetically encoded, i.e., a (glyco)protein amenable to molecular cloning. We then first constructed a hybrid protein by fusing the four extracellular Ig folds of EWI-2 to the constant domains of the hIg gamma heavy chain. This protein, termed sEWI-2-hIg, was purified and used as a FACS reagent to screen a broad panel of cell lines (Fig. 4). We found strong reactivity among T-cell leukemia lines, with Jurkat being consistently the most positive one. HUT78, Peer, and the epithelial line HeLa showed lower binding. Other T-cell lines, like CEM and primary peripheral blood T cells, all B-cell lines, and all lines of myeloid origin were negative.
FIG. 4.
Binding of sEWI-2-Ig fusion protein to various cell lines. Cells were incubated with 10 μg/ml sEWI-2-hIg, and binding was detected by anti-hIgG-FITC in a FACS. Percentages of cells positive above background are given.
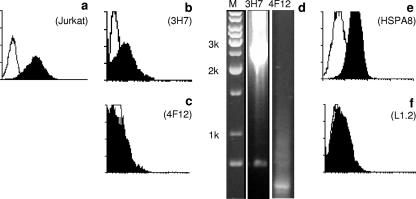
We therefore constructed a retroviral cDNA expression library from Jurkat cells and introduced this into SP2/0 cells. After several rounds of selection, individual clones were established and tested for binding of the sEWI-2 fusion protein, and the retroviral insert was retrieved from their genomic DNA (Fig. 5). Clone 3H7, which showed significant binding of the sEWI-2-hIg fusion protein compared to the control, harbored a complete, full-length transcript of the human HSPA8 variant 1 (accession number NM_006597) gene, including both the 5′ and 3′ untranslated regions. HSPA8 encodes a human HSP also known as hsc70 or Hsp73. To confirm this finding, an independent full-length HSPA8 cDNA was obtained from an unrelated source (IMAGE clone BC016660). This was cloned into a plasmid expression vector and introduced into murine L1.2 cells, and stable transfectants were established. Again, sEWI-2-hIg was found to bind these transfectants but not the parental L1.2 cells (Fig. 5e and f).
FIG. 5.
Identification of HSPA8 as the ligand of EWI-2. (a) sEWI-2-hIg (filled area) binds to Jurkat cells (empty profile, isotype control). (b and c) Binding of sEWI-2-hIg by two representative cell clones obtained after the combined magnetic/FACS selection procedure: clone 3H7 (b) bound sEWI-2-hIg, whereas 4F12 (c) was negative. (d) Nested PCR with vector-specific primers on genomic DNA isolated from the two respective single-cell clones. M, DNA site marker is base pairs. (e and f) Binding of sEWI-2-hIg to a L1.2 stable transfectant cell line expressing HSPA8 (e) or to the parental L1.2 cells (f).
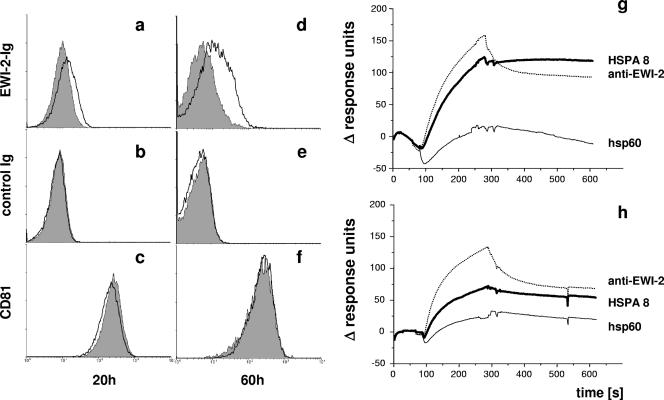
To confirm the role of HSPA8 in EWI-2 binding, we wanted to ablate the expression of HSPA8 on the surfaces of Jurkat cells. The appearance of HSPA8 at the cell surface (14) is regulated not only at the transcription/translation level but also by the release of HSPA8 protein molecules, which are then subsequently bound on the cell surface (4). We thus first introduced HSPA8-specific or control siRNA into Jurkat cells, and after allowing a short recovery, we further treated these cells with EDTA to strip surface-associated HSPA8. Cells treated with HSPA8-specific siRNA displayed only minimal sEWI-2-hIg binding (Fig. 6a and d). EWI-2 binding to Jurkat cells receiving control siRNA was low but significant at 20 h, but binding then substantially increased at 60 h. The binding of control proteins (Fig. 6b and e) and the expression of CD81 (Fig. 6c and f) remained essentially unaffected by the siRNA treatment.
FIG. 6.
HSPA8 is the ligand of EWI-2. (a to f) Jurkat cells were treated either with a combination of two HSPA8-specific siRNAs (gray shaded profiles) or with control siRNA (QIAGEN AllStars) (black empty histograms). Binding of sEWI-2-hIg (a and d) and of a control protein (b and e) and the expression of CD81 (c and f) were analyzed 20 h (left column [panels a, b, and c]) or 60 h (right column [panels d, e, and f]) after treatment. (g) Binding of 10 pM sEWI-2-hIg in fluid phase to the following BIACORE chip-immobilized proteins: human HSPA8, anti-EWI-2 mAb 5E8, and human hsp60. (h) The same experiment was performed in the presence of 10 μM ATP. One representative experiment out of three (each using independent protein preparations) is shown.
We then further characterized the HSPA8-EWI-2 interaction at the protein-protein level. Since HSPs constitute a broad family stretching across all living organisms, other homologues like human HSPA1 and HSPD1 and a mycobacterial hsp65 were tested in addition to human HSPA8 in BIACORE experiments. We detected binding of the sEWI-2-hIg fusion protein in the fluid phase towards immobilized HSPA8 with moderate affinity (Fig. 6g). This EWI-2-HSPA8 interaction was markedly but incompletely suppressed when the experiment was performed in the presence of 10 μM ATP (Fig. 6h). No binding of EWI-2 towards the other tested hsp homologues was seen; in addition, none of the immobilized HSPs bound an unrelated control fusion protein.
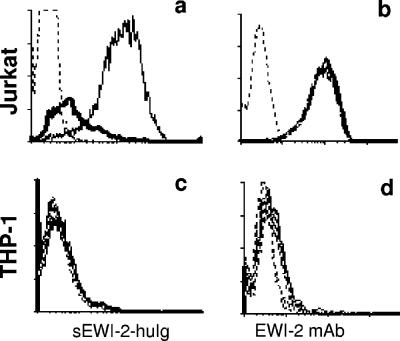
Since it is known that the surface levels of HSPA8 decrease after heat shock (4), one can expect that heat-treated HSPA8-carrying cells will lose or decrease their ability to bind sEWI-2-hIg. We thus tested Jurkat cells before and after heat shock and found that the binding of sEWI-2-hIg was significantly downregulated. At the same time, the expression of EWI-2 itself was not affected (Fig. 7a and b). The reactivity of THP-1 control cells remained unchanged (Fig. 7c and d).
FIG. 7.
Regulation of EWI-2/HSPA8 binding. (a) Heat shock treatment, which removes HSPA8 from the cell surface, results in decreased binding of sEWI-2-hIg to Jurkat cells. Thin line, binding at 37°C; thick line, binding after cells were treated for 30 min at 42°C; dashed line, isotype control. (b) The same treatment does not cause a change in the expression of EWI-2 itself on the surfaces of Jurkat cells. (c and d) THP-1 controls do not change their binding and expression levels.
The EWI-2-CD316 interaction modulates early DC responses.
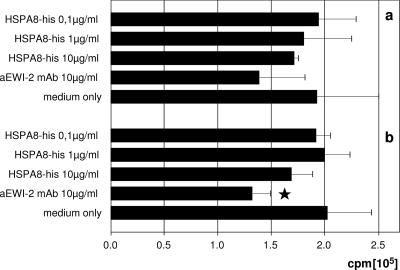
It has been extensively demonstrated previously that HSPs of every class and from diverse species enhance antigen uptake and presentation by DCs and that they can also promote cross-priming. Such properties were also demonstrated for the hsp70 proteins and also explicitly for HSPA8/hsc73 (15). However, an established role at the beginning of the DC activation phase does not exclude the possibility that HSPA8 exerts an additional regulatory function(s) during the ensuing subacute activation/postactivation phase. We thus wanted to characterize the role of the EWI-2-HSPA8 interaction on prestimulated DCs. We therefore generated mature DCs and activated and pulsed them with a defined allergen, Der p1, which is the major allergenic protein of the house dust mite Dermatophagoides pteronyssinus. After 24 h, DCs were extensively washed to remove antigen, and an aliquot from each donor was analyzed in a FACS to verify that the upregulation of EWI-2 also occurred. Pulsed DCs were then put into coculture with the Der p1-specific responder TCC 4:3.1 (5, 31) together with various concentrations of purified recombinant HSPA8 or anti-EWI-2 MAb. We found that HSPA8 incompletely but clearly dose dependently suppressed antigen-specific stimulation (Fig. 8). Notably, the addition of the anti-EWI-2 MAb 5E8 to the cultures had a similar suppressive effect.
FIG. 8.
Engaging EWI-2 on DCs diminishes antigen-driven proliferation. DCs were pulsed with 50 μg/ml Der p1 antigen peptide, washed, and placed into culture with 105 responder T cells of the clone 4:3.1. Various concentrations of recombinant HSPA8 or 10 μg/ml anti-EWI-2 mAb were added. Proliferations of responder T cells were determined 3 days later. Two independent donors (a and b) are shown; ★ indicates a P value of <0.05 in comparison to the medium control value.
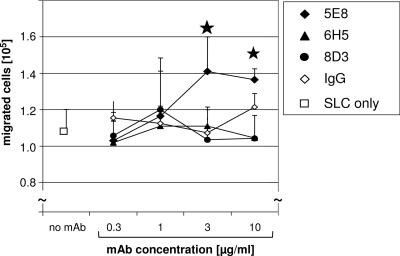
Two lines of literature data indicate that both EWI-2 and HSPA8 are integrated into widespread membrane protein clusters functionally connected to adhesion and migration: on the one hand, EWI-2 was shown to be part of a tetraspanin web dominated by CD9, CD81, and β1 integrins (11, 23); on the other hand, HSPA8 and the chemokine receptor CXCR4 are closely associated in a complex that can bind LPS (27). Therefore, we also tested whether signaling through EWI-2 would influence the migratory functions of DCs. We thus added anti-EWI-2 antibody to mature, activated DCs and surveyed the expression of TM4, the β1 integrin, and the chemokine receptor class members. The surface expression of the inspected molecules did not change upon the ligation of EWI-2 (data not shown). Cells were then placed into the Transwell upper chambers, and optimal concentrations of various chemokines were placed in the lower chambers. Test or control antibodies were added into both chambers. We found that MAb 5E8 significantly enhanced the migration of mature DCs against the CCR7 ligand SLC/CCL21, while two other nonfunctional anti-EWI-2 MAbs and a control antibody did not have any effect (Fig. 9). The EWI-2-mediated effects were specific for CCR7/CCL21, since the migration towards other chemokines, like SDF1/CXCL12, whose receptors are present on mature DCs, was not influenced (data not shown).
FIG. 9.
CCL21-induced migration of DCs is enhanced by ligation of EWI-2. Migration of mature DCs against 500 ng/ml SLC/CC21 was tested without antibodies added (1.09 × 105 ± 0.12 × 105 cells) or in the presence of various concentrations of the anti-EWI-2 mAbs 5E8, 6H5, and 8D3 or control IgG. Antibodies were present in both the upper and lower chambers. Numbers of migrated cells are given; ★ denotes a significant difference, with a P value of <0.05. Migration towards medium alone was 1.5 × 103 ± 1.5 × 103. Averages of quadruplicates from one representative donor (out of five) are shown.
DISCUSSION
The first physical barrier to microbiological invasion are the skin and mucosal membranes, and right beneath reside DCs (e.g., LCs in the dermis), which are the most potent professional antigen-presenting cells. But how and in what environment DCs encounter “danger” is decisive for the outcome of the emerging immune response, and this was the reason why we wanted to understand further details of the immediate early activation phase.
First, DCs receive (co)activation signals upon the recognition of PAMPs via TLRs. The responsiveness of PAMPs is linked to the expression of TLRs. We found that TLR1, TLR2, and TLR4 were highly expressed on MoDCs, whereas all other TLRs were expressed at very low levels. EWI-2 upregulation was observed using different TLR2 and TLR4 binding reagents, including LPS. Commercially available anti-TLR2- or TLR4-blocking antibodies or their combination (29) was only partially able to abrogate EWI-2 upregulation. One possible explanation is the known promiscuity of ligands binding several TLR receptors; another is that TLR2 forms heterodimers with either TLR1 or TLR6, either of which can maintain its function despite the antibody blockade (18). The molecular mechanisms by which TLRs induce gene expression are now rapidly being elucidated (25). Further experiments can thus precisely define how the induction of EWI-2 expression is connected to the signaling pathways downstream of TLRs.
The central molecule in this proposed early local feedback loop is EWI-2. EWI-2 was found by Charrin et al. (10) to be expressed in various immune cells, most prominently in unstimulated T, B, and NK cells, and we now identify EWI-2 as an activation marker of mature monocytes and DC subsets. Our time course expression experiments showed that EWI-2 is upregulated even earlier than CD86. A very similar expression pattern was also found for chemokines/cytokines and their receptors (28). Given the apparently synchronized kinetics of distinct functional groups, we propose that EWI-2 is part of a broader “early regulatory” system and that interactions with or through EWI-2 influence or fine-tune the final outcome of the DC maturation process.
For transmitting a signal, an external ligand is required. Three lines of evidence indicate that HSPA8 is a ligand of EWI-2: (i) direct expression cloning, (ii) genetic complementation (transfection) of a nonbinder host cell line by use of an independent HSPA8 encoding cDNA and loss of EWI-2 binding upon HSPA8-specific siRNA treatment, and (iii) direct demonstration of protein-protein interaction. HSPA8 has been described as an intracellular chaperone that is present in the cytosols of all prokaryotic and eukaryotic organisms. Although the HSPA8 molecule lacks both a signal sequence and a transmembrane domain, HSPA8 was found to be overexpressed on the cell surface by many different cells, including leukemia lines, thyroid cancer cells, and primary melanoma cells (4, 12). HSPs from other classes, including HSP60 (7) and gp96, were also found at the cell surface.
In recent years, several hsp70 receptors, including CD14, TLR2, TLR4, CD40, CD91, and LOX-1 have been identified (8). The LPS receptors CD14 and TLR4 were implicated in binding hsp60/65 (16, 20). These receptors were also reported to bind the human hsp70, but the significance of these findings is under debate, as CD14 and TLR2/4 are also simultaneously LPS receptors. CD91 has also been shown to be a receptor for hsp70, hsp90, and calreticulin (6). Due to its large size, however, no experiments were performed that would directly have confirmed the binding of hsp70 to CD91-transfected cells. LOX-1, another scavenger receptor, was identified as a receptor for endocytic uptake of the hsp70 class and their chaperoned peptides by human DCs (13). In contrast to EWI-2, LOX-1 is present on immature DCs. These data can be interpreted to indicate that different hsp receptors might have correspondingly different functions: the big, multipurpose scavenger-type receptors, like LOX1 and CD91, are involved in the early phase, when antigen uptake is facilitated by hsp (26), and are also responsible for the delivery of hsp-associated antigens for presentation (19), while the ligand-specific inducible receptors, like EWI-2, would have primarily regulatory roles. The most unique property of EWI-2 is that it seems to have a restricted repertoire of hsp ligands or at least displays a certain preference in this regard: we found exclusive binding towards HSPA8, while the homologous HSPA1 and HSPD1 and the unrelated mycobacterial hsp65 were not recognized. Although the overall structure of the hsp70 class is generally highly conserved, it is important to note that there are significant amino acid differences between HSPA8 and other family members. We mapped HSPA8 to a consensus model based on the 1YUW and 1S3X crystal structures representing HSPA1 and found that there is a prominent string of divergent amino acids near the peptide binding domain. In addition, variations in conformation could also account for selectivity. ATP, which causes such changes, was indeed interfering with the EWI-2-HSPA8 interaction.
We hypothesized that rapid changes in the gene expression pattern of DCs after the initial activation would lead to the appearance of new surface receptors, which in turn could offer a possibility for in situ fine-tuning of DC behavior. In this study we (i) identified EWI-2/CD316 as one of the earliest molecules upregulated upon activation on several subtypes of DCs; (ii) showed that HSPA8 is a ligand for EWI-2; and (iii) demonstrated that through EWI-2-mediated interactions, the migratory and antigen-presenting capacities of DCs are altered. In conclusion, interactions of EWI-2 with its newly identified ligand, HSPA8, may have multiple opportunities to influence the downstream behavior of DCs and thereby control the ensuing adaptive immune response. Interfering with this pathway therefore may have possible therapeutic utility in the treatment of autoimmune diseases. It will be important to clarify the contribution of this pathway in pathological settings, especially in situations where both high levels of HSPA8 and local accumulation of DCs were observed, like in rheumatoid arthritis.
Acknowledgments
We thank Markus Jaritz for his help with bioinformatics; Christine Ruf for technical assistance; and Thomas Baumruker, Hannes Stockinger, Herbert Strobl, and Antal Rot for advice and discussions.
Footnotes
Published ahead of print on 4 September 2007.
REFERENCES
- 1.Akagi, T., K. Sasai, and H. Hanafusa. 2003. Refractory nature of normal human diploid fibroblasts with respect to oncogene-mediated transformation. Proc. Natl. Acad. Sci. USA 100: 13567-13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18: 767-811. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392: 245-252. [DOI] [PubMed] [Google Scholar]
- 4.Barreto, A., J. M. Gonzalez, E. Kabingu, A. Asea, and S. Fiorentino. 2003. Stress-induced release of HSC70 from human tumors. Cell. Immunol. 222: 97-104. [DOI] [PubMed] [Google Scholar]
- 5.Baselmans, P. J., E. Pollabauer, F. C. van Reijsen, H. C. Heystek, A. Hren, P. Stumptner, M. G. Tilanus, W. C. Vooijs, and G. C. Mudde. 2000. IgE production after antigen-specific and cognate activation of HLA-DPw4-restricted T-cell clones, by 78% of randomly selected B-cell donors. Hum. Immunol. 61: 789-798. [DOI] [PubMed] [Google Scholar]
- 6.Basu, S., R. J. Binder, T. Ramalingam, and P. K. Srivastava. 2001. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14: 303-313. [DOI] [PubMed] [Google Scholar]
- 7.Belles, C., A. Kuhl, R. Nosheny, and S. R. Carding. 1999. Plasma membrane expression of heat shock protein 60 in vivo in response to infection. Infect. Immun. 67: 4191-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binder, R. J., R. Vatner, and P. Srivastava. 2004. The heat-shock protein receptors: some answers and more questions. Tissue Antigens 64: 442-451. [DOI] [PubMed] [Google Scholar]
- 9.Caux, C., C. Dezutter-Dambuyant, D. Schmitt, and J. Banchereau. 1992. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature 360: 258-261. [DOI] [PubMed] [Google Scholar]
- 10.Charrin, S., F. Le Naour, V. Labas, M. Billard, J. P. Le Caer, J. F. Emile, M. A. Petit, C. Boucheix, and E. Rubinstein. 2003. EWI-2 is a new component of the tetraspanin web in hepatocytes and lymphoid cells. Biochem. J. 373: 409-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charrin, S., F. Le Naour, M. Oualid, M. Billard, G. Faure, S. M. Hanash, C. Boucheix, and E. Rubinstein. 2001. The major CD9 and CD81 molecular partner. Identification and characterization of the complexes. J. Biol. Chem. 276: 14329-14337. [DOI] [PubMed] [Google Scholar]
- 12.Deichmann, M., M. Polychronidis, A. Benner, C. Kleist, M. Thome, B. Kahle, and B. M. Helmke. 2004. Expression of the heat shock cognate protein HSP73 correlates with tumour thickness of primary melanomas and is enhanced in melanoma metastases. Int. J. Oncol. 25: 259-268. [PubMed] [Google Scholar]
- 13.Delneste, Y., G. Magistrelli, J. F. Gauchat, J. F. Haeuw, J. P. Aubry, K. Nakamura, N. Kawakami-Honda, L. Goetsch, T. Sawamura, J. Y. Bonnefoy, and P. Jeannin. 2002. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity 17: 353-362. [DOI] [PubMed] [Google Scholar]
- 14.Hantschel, M., K. Pfister, A. Jordan, R. Scholz, R. Andreesen, G. Schmitz, H. Schmetzer, W. Hiddemann, and G. Multhoff. 2000. Hsp70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones 5: 438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kammerer, R., D. Stober, P. Riedl, C. Oehninger, R. Schirmbeck, and J. Reimann. 2002. Noncovalent association with stress protein facilitates cross-priming of CD8+ T cells to tumor cell antigens by dendritic cells. J. Immunol. 168: 108-117. [DOI] [PubMed] [Google Scholar]
- 16.Kol, A., A. H. Lichtman, R. W. Finberg, P. Libby, and E. A. Kurt-Jones. 2000. Heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 164: 13-17. [DOI] [PubMed] [Google Scholar]
- 17.Kolesnikova, T. V., C. S. Stipp, R. M. Rao, W. S. Lane, F. W. Luscinskas, and M. E. Hemler. 2004. EWI-2 modulates lymphocyte integrin {alpha}4{beta}1 functions. Blood 103: 3013-3019. [DOI] [PubMed] [Google Scholar]
- 18.Lee, J. Y., L. Zhao, H. S. Youn, A. R. Weatherill, R. Tapping, L. Feng, W. H. Lee, K. A. Fitzgerald, and D. H. Hwang. 2004. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J. Biol. Chem. 279: 16971-16979. [DOI] [PubMed] [Google Scholar]
- 19.Noessner, E., R. Gastpar, V. Milani, A. Brandl, P. J. Hutzler, M. C. Kuppner, M. Roos, E. Kremmer, A. Asea, S. K. Calderwood, and R. D. Issels. 2002. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J. Immunol. 169: 5424-5432. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 164: 558-561. [DOI] [PubMed] [Google Scholar]
- 21.Schett, G., K. Redlich, Q. Xu, P. Bizan, M. Groger, M. Tohidast-Akrad, H. Kiener, J. Smolen, and G. Steiner. 1998. Enhanced expression of heat shock protein 70 (hsp70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. Differential regulation of hsp70 expression and hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J. Clin. Investig. 102: 302-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son, Y. S., J. H. Park, Y. K. Kang, J. S. Park, H. S. Choi, J. Y. Lim, J. E. Lee, J. B. Lee, M. S. Ko, Y. S. Kim, J. H. Ko, H. S. Yoon, K. W. Lee, R. H. Seong, S. Y. Moon, C. J. Ryu, and H. J. Hong. 2005. Heat shock 70-kDa protein 8 isoform 1 is expressed on the surface of human embryonic stem cells and downregulated upon differentiation. Stem Cells 23: 1502-1513. [DOI] [PubMed] [Google Scholar]
- 23.Stipp, C. S., T. V. Kolesnikova, and M. E. Hemler. 2001. EWI-2 is a major CD9 and CD81 partner and member of a novel Ig protein subfamily. J. Biol. Chem. 276: 40545-40554. [DOI] [PubMed] [Google Scholar]
- 24.Stipp, C. S., T. V. Kolesnikova, and M. E. Hemler. 2003. EWI-2 regulates alpha3beta1 integrin-dependent cell functions on laminin-5. J. Cell Biol. 163: 1167-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda, K., and S. Akira. 2004. TLR signaling pathways. Semin. Immunol. 16: 3-9. [DOI] [PubMed] [Google Scholar]
- 26.Theriault, J. R., H. Adachi, and S. K. Calderwood. 2006. Role of scavenger receptors in the binding and internalization of heat shock protein 70. J. Immunol. 177: 8604-8611. [DOI] [PubMed] [Google Scholar]
- 27.Triantafilou, K., M. Triantafilou, and R. L. Dedrick. 2001. A CD14-independent LPS receptor cluster. Nat. Immunol. 2: 338-345. [DOI] [PubMed] [Google Scholar]
- 28.Tureci, O., H. Bian, F. O. Nestle, L. Raddrizzani, J. A. Rosinski, A. Tassis, H. Hilton, M. Walstead, U. Sahin, and J. Hammer. 2003. Cascades of transcriptional induction during dendritic cell maturation revealed by genome-wide expression analysis. FASEB J. 17: 836-847. [DOI] [PubMed] [Google Scholar]
- 29.Uehori, J., M. Matsumoto, S. Tsuji, T. Akazawa, O. Takeuchi, S. Akira, T. Kawata, I. Azuma, K. Toyoshima, and T. Seya. 2003. Simultaneous blocking of human Toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus Calmette-Guérin peptidoglycan. Infect. Immun. 71: 4238-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Hoff, M. J., A. F. Moorman, and W. H. Lamers. 1992. Electroporation in ‘intracellular’ buffer increases cell survival. Nucleic Acids Res. 20: 2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Reijsen, F. C., C. A. Bruijnzeel-Koomen, F. S. Kalthoff, E. Maggi, S. Romagnani, J. K. Westland, and G. C. Mudde. 1992. Skin-derived aeroallergen-specific T-cell clones of Th2 phenotype in patients with atopic dermatitis. J. Allergy Clin. Immunol. 90: 184-193. [DOI] [PubMed] [Google Scholar]
- 32.Vidal-Laliena, M., X. Romero, S. March, V. Requena, J. Petriz, and P. Engel. 2005. Characterization of antibodies submitted to the B cell section of the 8th Human Leukocyte Differentiation Antigens Workshop by flow cytometry and immunohistochemistry. Cell. Immunol. 236: 6-16. [DOI] [PubMed] [Google Scholar]
- 33.Wellenreuther, R., I. Schupp, A. Poustka, and S. Wiemann. 2004. SMART amplification combined with cDNA size fractionation in order to obtain large full-length clones. BMC Genomics 5: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, X. A., W. S. Lane, S. Charrin, E. Rubinstein, and L. Liu. 2003. EWI2/PGRL associates with the metastasis suppressor KAI1/CD82 and inhibits the migration of prostate cancer cells. Cancer Res. 63: 2665-2674. [PubMed] [Google Scholar]