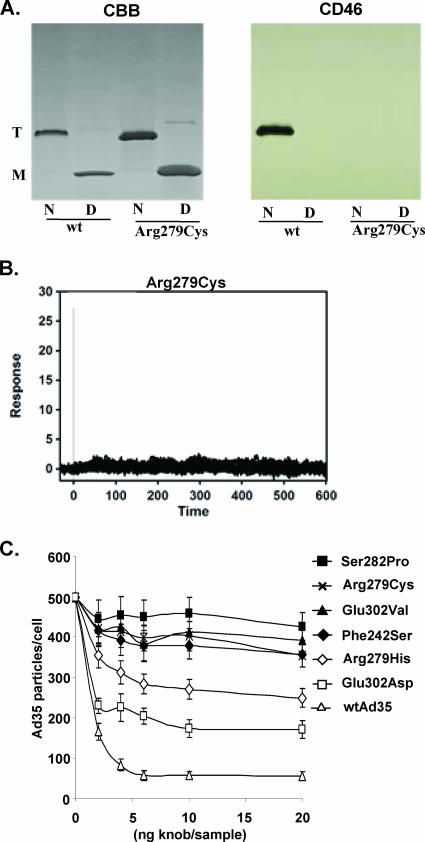
FIG. 3.
Analysis of functional properties of selected Ad35 knob mutants. (A) Binding of Arg279Cys mutant to sCD46 in Western blot analysis. Purified Ad35 wild-type and mutant knobs were run as native (N) or as denatured (D) protein on a polyacrylamide gel. Coomassie brilliant blue staining (CBB) revealed the trimeric knob form (T), which is converted into monomers after boiling (M). Blots were analyzed for CD46 binding by subsequent incubation with sCD46, anti-CD46 MAb, and anti-mouse IgG-HRP. (B) Biacore response data for sCD46 binding to biosensor surface containing Arg297Cys knob (127 RU). Experimental data represent the responses of duplicate injections of various concentrations of sCD46 (169 nM, 56 nM, 19 nM, 6 nM, and 2 nM). Experimental conditions were the same as for wild-type Ad35 knob (Fig. 1B). (C) Virus binding competition assay. A total of 1 × 105 HeLa cells were preincubated with different concentrations of recombinant Ad35 fiber knobs at 4°C for 1 h before incubation with [3H]thymidine-labeled wild-type Ad35 (wtAd35) at a multiplicity of infection of 8,000 VP/cell for another hour, and the number of VP bound per cell was determined after washing with PBS.