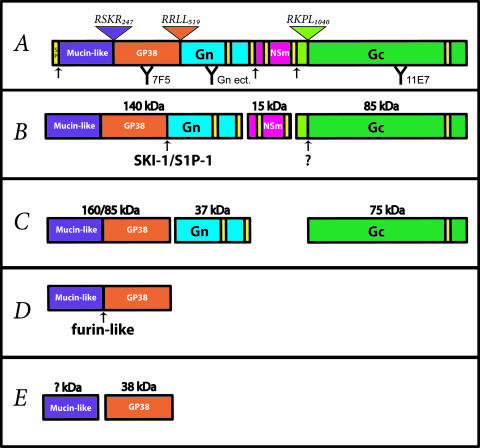
FIG. 1.
Schematic representation of CCHFV M-encoded polyprotein domains and proteolytic processing. (A) Signal peptide (SP), mucin-like, GP38, Gn, NSm, and Gc M polyprotein domains are highlighted. Potential transmembrane domains (yellow) and signal peptidase cleavage sites are indicated by black arrows. Antibodies used in this study are indicated (with their binding regions in parentheses): 7F5 (PreGn/GP38), Gn ectodomain (Gn ect.) (Gn), and 11E7 (PreGc/Gc). Defined furin-like (RSKR247), SKI-1/S1P (RRLL519), and SKI-1/S1P-like (RKPL1040) cleavage sites are illustrated by inverted triangles. (B) The first proteolytic products are expected to occur cotranslationally or rapidly after the synthesis of the polyprotein. PreGn (140 kDa), NSm (15 kDa), and PreGc (85 kDa) are the results of these initial processing events. SKI-1/S1P and PreGc convertase will then cleave (indicated by arrows) PreGn and PreGc in the early secretory pathway. The PreGc cleavage also occurs early in the secretory pathway but the cognate protease (?) remains unidentified. (C) The activity of SKI-1/S1P and the PreGc convertase generates a nonstructural mucin-like GP38 protein of either 160 or 85 kDa, and the structural glycoproteins Gn (37 kDa) and Gc (75 kDa). (D) The mucin-like GP38 is further cleaved (arrow) by a furin-like protease in the late secretory pathway. (E) Furin-like enzyme cleavage results in production of a GP38 glycoprotein (38 kDa) and a mucin-like protein of unknown mass (? kDa).