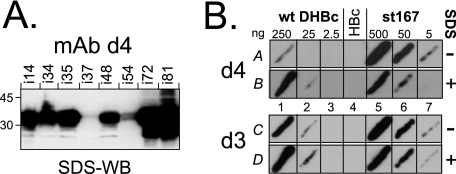
FIG. 2.
The epitope of MAb d4 is bipartite and only accessible in nonassembled DHBc. (A) Epitope mapping by SDS-WB of DHBc insertion variants. Similar amounts of various mutant DHBc proteins were analyzed by SDS-WB with MAb d4. All tested peptide insertion variants were well detected except the insertion mutants i37 and i54. A peptide insertion at position 48 (i48) led to an only modest reduction in signal intensity. (B) The MAb d4 epitope is hidden in intact DHBc particles. Aliquots of native lysates containing wt-DHBc and the nonassembling variant st167 were applied in three 10-fold dilutions containing the indicated amounts of antigen; the single HBc control streak contained about 500 ng of core protein. Membrane strip A was directly processed, strip B was first treated for 10 min at 50°C in 0.1% SDS solution. wt-DHBc reactivity was enhanced ∼10-fold by SDS treatment (A1 versus B1), whereas st167, if anything, was more efficiently recognized without than with SDS treatment (A5 versus B5). No assembly status-associated differences were detected with MAb d3 (lower panel, fields C and D).