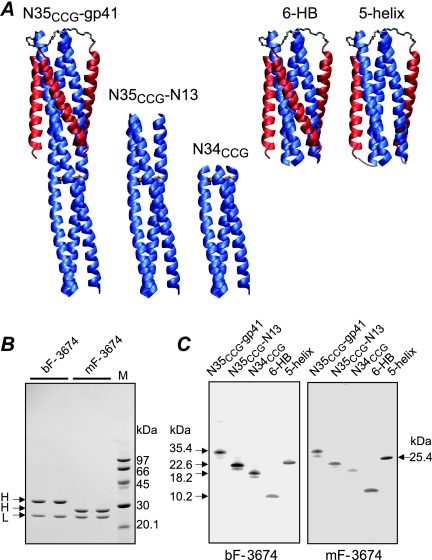
FIG. 1.
Characterization of bivalent (bF) and monovalent (mF) Fab 3674 antibodies. (A) Ribbon diagrams of the engineered gp41-derived constructs used in this study. The N-HR and C-HR helices are displayed in blue and red, respectively, and disulfide bridges linking the N-HR helices (NCCG constructs) are shown as gold rods. (B) SDS-PAGE of reduced bF-3674 and mF-3674 stained with Coomassie. H and L denote heavy and light chains, respectively. Molecular masses of protein standards are indicated. The larger mass of the heavy chain of bF-3674 relative to that for mF-3674 is due to the helix-loop-helix dimerization domain. (C) Western blot analysis under nonreducing conditions of gp41-derived constructs reacting with bF-3674 and mF-3674. Molecular masses are indicated. Disulfide-linked NCCG-gp41, N35CCG-N13, and N34CCG refold spontaneously, just like the 6-HB. Binding is conformation dependent as reduced forms of NCCG-gp41, N35CCG-N13, and N34CCG, which do not refold spontaneously but form heterogeneous aggregates due to the exposed N-HR region (23, 24) not conformationally restricted by disulfide bridges, do not interact with the Fabs.