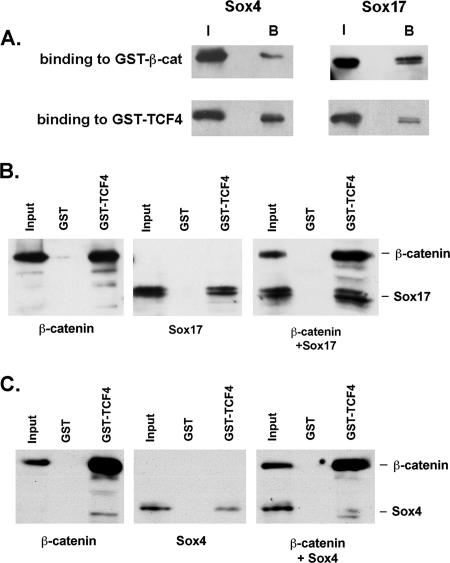
FIG. 5.
Sox4 and Sox17 have similar affinities for TCF4 and β-catenin, but Sox4 does not form a stable complex. (A) Sox4 has affinities for TCF4 and β-catenin (β-cat) similar to those of Sox17. The ratios of the input (I) and bound (B) protein fractions are similar for Sox17 and Sox4. (B) Sox17 formed a complex with β-catenin and TCF4. Also, more Sox17 protein was pulled down by TCF4-GST beads in the presence of β-catenin. (C) GST-TCF4 beads were able to pull down either β-catenin or Sox4 but did not efficiently pull down Sox4 in the presence of β-catenin. Moreover, Sox4 binding to TCF4 was somewhat inhibited in the presence of β-catenin, suggesting that Sox4 and β-catenin compete for binding.