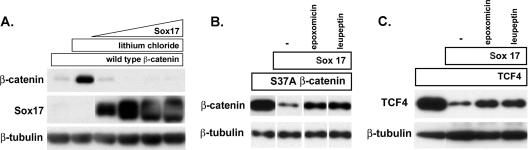
FIG. 8.
Sox17-mediated degradation of β-catenin and TCF4 is independent of GSK3β activity and mediated by the proteasome. (A) LiCl does not affect Sox17-mediated degradation of β-catenin. COS cells were transfected with an expression plasmid encoding wild-type β-catenin (100 ng; myc tagged) in the absence (lane 1) or presence(lanes 2 to 6) of 40 mM LiCl. Cells were cotransfected with increasing amounts of Sox17 plasmid (50, 100, 200, and 400 ng) and the wild-type β-catenin gene in the presence of lithium chloride (lanes 3 to 6). LiCl effectively stabilized wild-type β-catenin protein in the absence of Sox17 but could not inhibit Sox17-mediated degradation of wild-type β-catenin protein. (B and C) The proteasome inhibitor epoxomicin inhibits Sox17-induced degradation of stabilized β-catenin (B) and TCF4 (C). Cells were transfected with the β-catenin(S37A) mutant form (100 ng) or TCF4 (100 ng) alone or together with Sox17 (100 ng). Culturing cotransfected cells with epoxomicin (0.1 μM) or leupeptin (25 μM) inhibited Sox17-mediated degradation of β-catenin and TCF4 proteins. The lysosomal acidification inhibitor cholorquine (25 μM) had no effect (data not shown). −, control.