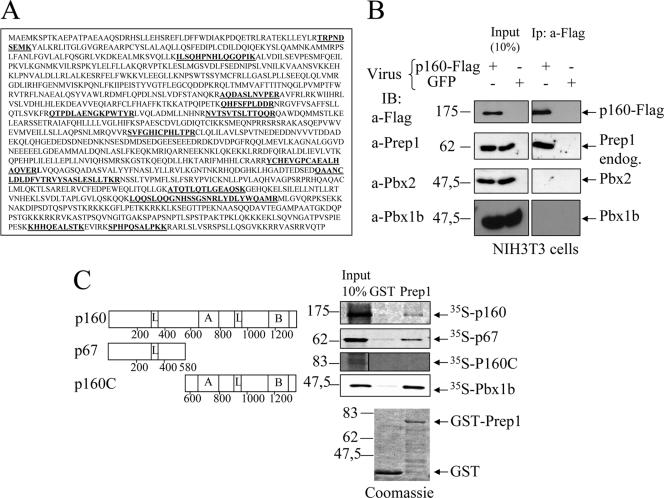
FIG. 1.
In vivo and in vitro interactions of Prep1 and p160. (A) The sequence of mouse p160. In boldface are the peptides identified by matrix-assisted laser desorption ionization-time of flight after tandem-affinity purification (11). (B) Immunoprecipitation with M2 anti-FLAG antibody and immunoblotting with the indicated antibodies of nuclear extracts of NIH 3T3 cells infected with p160-FLAG or a control GFP retrovirus as indicated. Input and immunoprecipitates (Ip) are compared (see Materials and Methods). (C) Schematic representation of p160 deletion mutants (left) and binding to recombinant GST-Prep1 (right). The leucine zipper-like motifs (L), the acidic domain (A), and the basic carboxyl-terminal region (B) are indicated. Binding was performed with in vitro-translated 35S-p160, 35S-p67, 35S-p160C, and 35S-Pbx1b. On the upper right is shown autoradiography of different pull downs performed with either GST (control) or GST-Prep1 (Prep1) and the 35S-labeled proteins. On the bottom are the Coomassie-stained gels of GST and GST-Prep1.