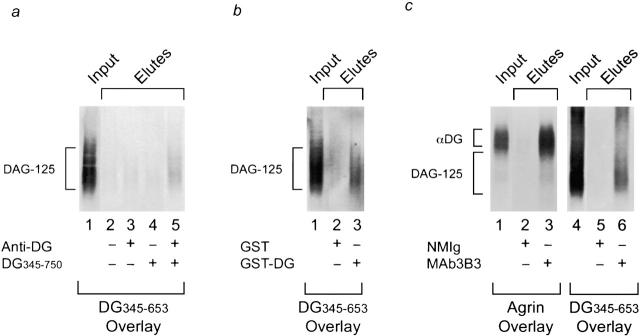
Figure 2.
Coprecipitation of DAG-125 and dystroglycan (αDG). DAG-125 was solubilized by alkaline treatment, neutralized, and incubated with column matrices and recombinant or native dystroglycan as indicated. The input material and eluates from the beads were analyzed by ligand blot overlay assay for the presence of DAG-125 (35S-DG345-653 as probe) or native α-dystroglycan (agrin overlay, see Materials and Methods). a, Coimmunoprecipitation of DAG-125 and in vitro synthesized dystroglycan. DAG-125 was incubated with goat anti–mouse Ig-conjugated agarose beads in the presence or absence of in vitro translated dystroglycan polypeptide (DG345-750) and/or antidystroglycan mAb (NCL-β-DG; Novocastra). DAG-125 coprecipitated with dystroglycan plus antidystroglycan antibody (lane 5), but was not precipitated in the absence of either or both (lanes 2–4). b, Coaffinity precipitation of DAG-125 and bacterially produced dystroglycan. DAG-125 was incubated with glutathione Sepharose beads that had been preincubated with either bacterially produced GST or a bacterially produced GST–dystroglycan fusion protein (GST–DG345-653). DAG-125 was coprecipitated with the dystroglycan fusion protein (lane 3), but not with GST alone (lane 2). c, Coimmunoprecipitation of DAG-125 and native α-dystroglycan. DAG-125 and α-dystroglycan were solubilized by alkaline treatment, and incubated with agarose beads conjugated to either normal mouse Ig (NMIg) or anti-Torpedo dystroglycan mAb, mAb3B3. Native α-dystroglycan and DAG-125 were coprecipitated by the antidystroglycan antibody (lanes 3 and 6), but not by control antibody (lanes 2 and 5). Western blots indicate that mAb3B3 does not recognize DAG-125 (data not shown; see Bowe et al. 1994).