Figure 4.
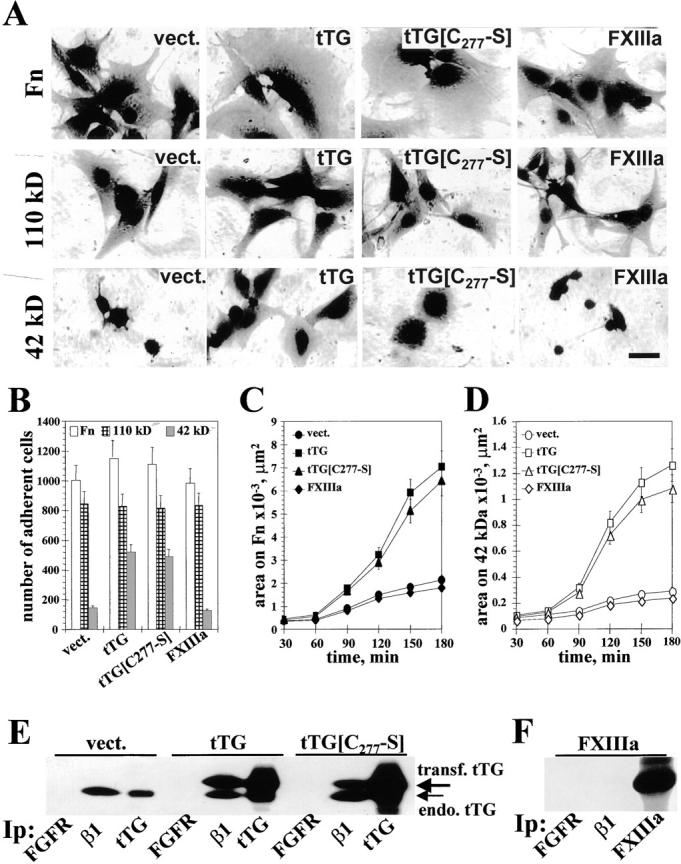
tTG-dependent stimulation of cell adhesion and spreading on Fn and its 42-kD fragment does not require the cross-linking activity. (A) Spreading assays with REF52 transfectants. Cells expressing vector, tTG, tTG[C277→S], or FXIIIa were plated for 4 h on Fn, 110-kD, or 42-kD Fn fragments. Bar, 20 μM. (B) Quantitative adhesion assays with REF52 transfectants plated for 1 h on Fn (open bars), 110-kD (crossed bars), and 42-kD (filled bars) fragments. Shown are the means of quadruplicate measurements. (C and D) Quantitative spreading assays with REF52 transfectants. Cells expressing vector, tTG, tTG[C277→S], or FXIIIa were plated for 30–180 min on Fn (C) or the 42-kD Fn fragment (D). Shown are the average areas on the substrates for 90 cells. (A–D) Cells were plated for adhesion and spreading assays in serum-free cycloheximide-containing medium. (E) Analysis of tTG association with β1 integrins. FGF receptor, β1 integrins, and tTG were immunoprecipitated with polyclonal antibody Flg(H-76) or HMβ1-1 and CUB7402 mAbs, respectively, from REF52 cells expressing vector, tTG or tTG[C277→S]. The resulting immunoprecipitates were blotted for tTG. Large arrow marks the transfected human tTG and small arrow indicates the endogenous rat tTG. (F) FXIIIa is not associated with β1 integrins. FGF receptor, β1 integrins, and FXIIIa were immunoprecipitated from FXIIIa-expressing REF52 cells and the resulting immunoprecipitates were probed for FXIIIa with a polyclonal antibody.
