Figure 7.
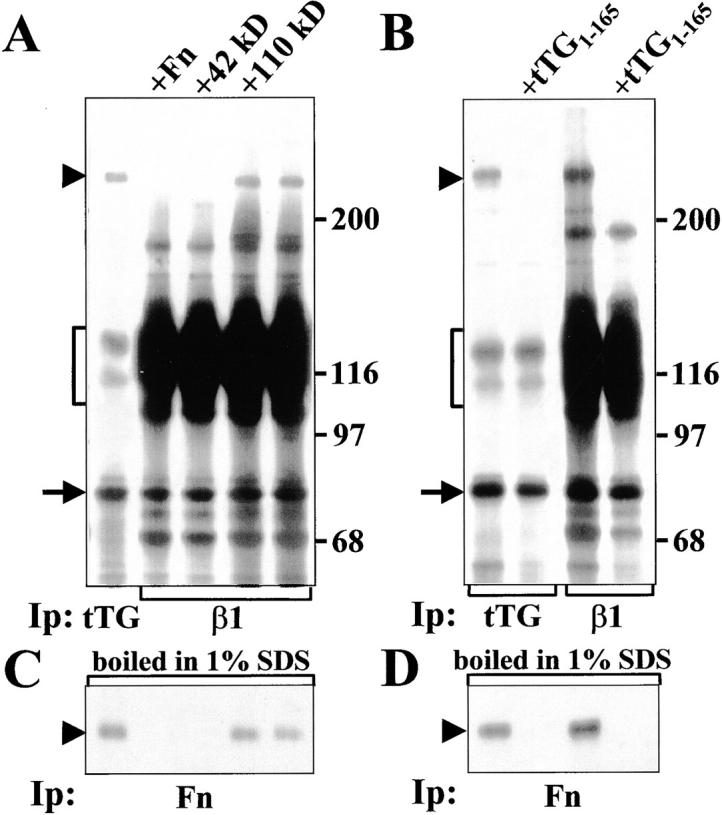
Interaction of tTG with β1 integrins allows formation of ternary complexes with Fn. (A) tTG (left lane) or β1 integrins (all other lanes) were immunoprecipitated from RIPA lysates of 35S-labeled WI-38 fibroblasts either in the presence of 1 μM unlabeled Fn, its 42-kD fragment, its 110-kD fragment or without any of these proteins added. (B) tTG (left two lanes) or β1 integrins (right two lanes) were immunoprecipitated from RIPA lysates of 35S-labeled WI-38 fibroblasts either in the absence or with 5 μM unlabeled NH2-terminal tTG fragment tTG1-165. After immunoprecipitation half of each sample shown in A and B was boiled in 1% SDS, reconstituted with 10 volumes of 1% Triton X-100 in TBS and subjected to reprecipitation with polyclonal antibody against Fn (C and D). Note a disappearance of 35S-labeled Fn bands in the samples treated with excess unlabeled Fn, 42-kD Fn fragment, or tTG1-165, but not with excess unlabeled 110-kD Fn fragment. Arrowheads indicate Fn bands. Brackets mark α5β1 integrin. Arrows point to tTG bands. Molecular weight markers are shown to the right of the gels.
