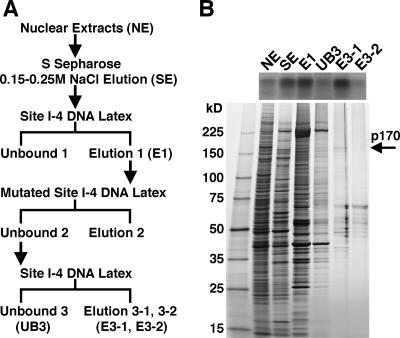
FIG. 2.
Identification of factor I-4 as a Blimp-1/CG5249-encoded protein. (A) Purification of factor I-4. Nuclear extract (NE) derived from embryos at 8 to 16 h after egg laying was loaded onto an S Sepharose column. Fractions eluted between 0.15 and 0.25 M NaCl (SE) were subjected to affinity purification. The first affinity purification was performed using site I-4 DNA-conjugated latex beads. The eluate from the first affinity chromatography (E1) was then incubated with mutated site I-4 DNA-conjugated beads to perform subtraction. The supernatant from the subtraction was subjected to a second affinity purification. Eluate from the second affinity chromatography (E3-1 and E3-2) was obtained. (B) Detection of the binding activity and proteins in typical fractions during purification by a gel mobility shift assay (upper panel) and SDS-PAGE (lower panel). One microliter of the fractions in the purification step was used for the gel mobility shift assay, and 10 μl of the same fraction was reserved for SDS-PAGE, except for NE and SE, which were loaded at only 0.5 μl. Proteins were detected by silver staining.