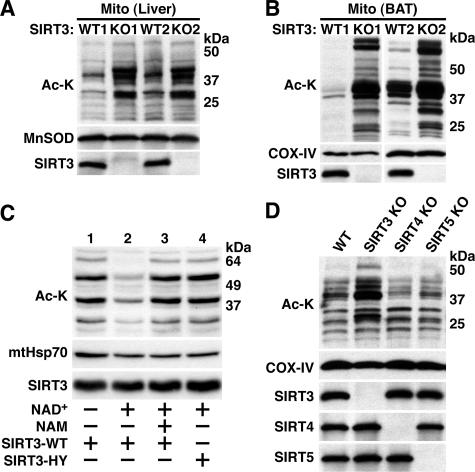
FIG. 3.
SIRT3 is a major mitochondrial protein deacetylase in vivo. (A) Hyperacetylation of mitochondrial proteins in liver of SIRT3-deficient mice. SIRT3-deficient and littermate control mitochondrial extracts from two mice per genotype were fractionated by SDS-PAGE and immunoblotted with polyclonal antibodies to acetyl-lysine (Ac-K), SIRT3, or MnSOD as a loading control. (B) Hyperacetylation of mitochondrial proteins in BAT of SIRT3-deficient mice. Mitochondrial extracts from two SIRT3-deficient mice and two wild-type littermate controls were probed as in panel A except that COX-IV was used as a loading control. (C) Recombinant SIRT3 reverses mitochondrial hyperacetylation associated with SIRT3 deficiency. Mitochondrial extracts were treated with recombinant wild-type (WT) SIRT3 or a SIRT3 catalytic mutant (SIRT3-HY) as shown. NAD+, a cofactor required for sirtuin-mediated deacetylation, and nicotinamide (NAM), a sirtuin inhibitor, were added as indicated. Samples were separated by SDS-PAGE and probed with a monoclonal acetyl-lysine antibody. SIRT3 antibodies were used to demonstrate the presence of recombinant SIRT3; mtHsp70 served as a loading control. (D) SIRT3, but not SIRT4 or SIRT5, is responsible for global protein deacetylation in mitochondria. Liver mitochondrial extracts were generated from mice of the indicated genotypes and analyzed as in panel A.