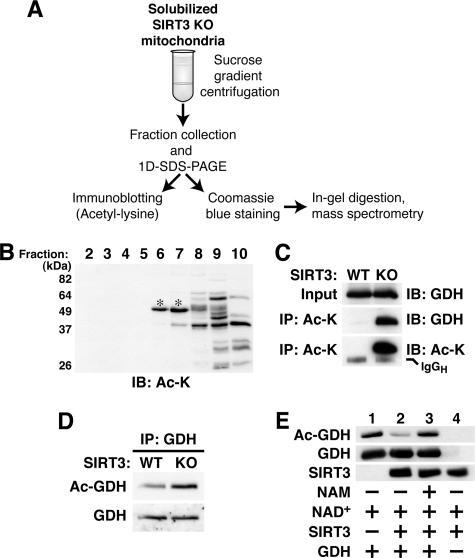
FIG. 4.
SIRT3 deacetylates GDH in vivo. (A) Scheme for identifying putative SIRT3 substrates from mouse liver mitochondria. KO, knockout. (B) Representative immunoblot (IB) of protein fractions prepared as illustrated in panel A and probed with monoclonal acetyl-lysine antibody. *, acetyl-lysine antibody-reactive bands analyzed by mass spectrometry. (C and D) GDH is hyperacetylated in SIRT3-deficient mouse liver mitochondria. (C) Acetylated proteins from liver mitochondria of animals with the indicated genotypes were immunoprecipitated (IP) with monoclonal acetyl-lysine antibodies, fractionated by SDS-PAGE, and probed with GDH antibodies. Results shown are representative of five independent experiments. (D) GDH immune complexes from wild-type and SIRT3-deficient liver mitochondria were immunoblotted with monoclonal acetyl-lysine antibodies (Ac-GDH). Probing with GDH antibodies revealed total GDH levels. Results shown are representative of three independent experiments. (E) GDH is a bona fide SIRT3 substrate. Bovine GDH was incubated with recombinant SIRT3, NAD+, and nicotinamide (NAM) as indicated. Reactions were stopped by boiling in SDS sample buffer, and levels of total and acetylated GDH were analyzed by immunoblotting. The presence of recombinant SIRT3 was demonstrated by immunoblotting with antibodies to SIRT3.