Abstract
No cytochrome pigments were detected by difference (reduced minus oxidized) spectroscopy at liquid nitrogen temperature in whole-cell preparations or membrane fractions of Acholeplasma axanthum S273, Acholeplasma equifetale N93, Acholeplasma granularum BTS39, Acholeplasma laidlawii B-PG9, Acholeplasma modicum PG-49, Acholeplasma oculi 19L, Mycoplasma arginini G230, Mycoplasma arthritidis 07, Mycoplasma pneumoniae FH, and Mycoplasma pulmonis JB. All ten Mollicutes species examined contained iron of unknown function (3.0 to 15.3 nmol of iron per mg of protein). Relatively small amounts of acid-labile sulfide were found in all fractions (0.10 to 1.07 nmol of acid-labile sulfide per mg of protein). The data suggest that, as Mollicutes lack cytochrome pigments, they would synthesize most if not all adenosine triphosphate at the substrate level.
Full text
PDF

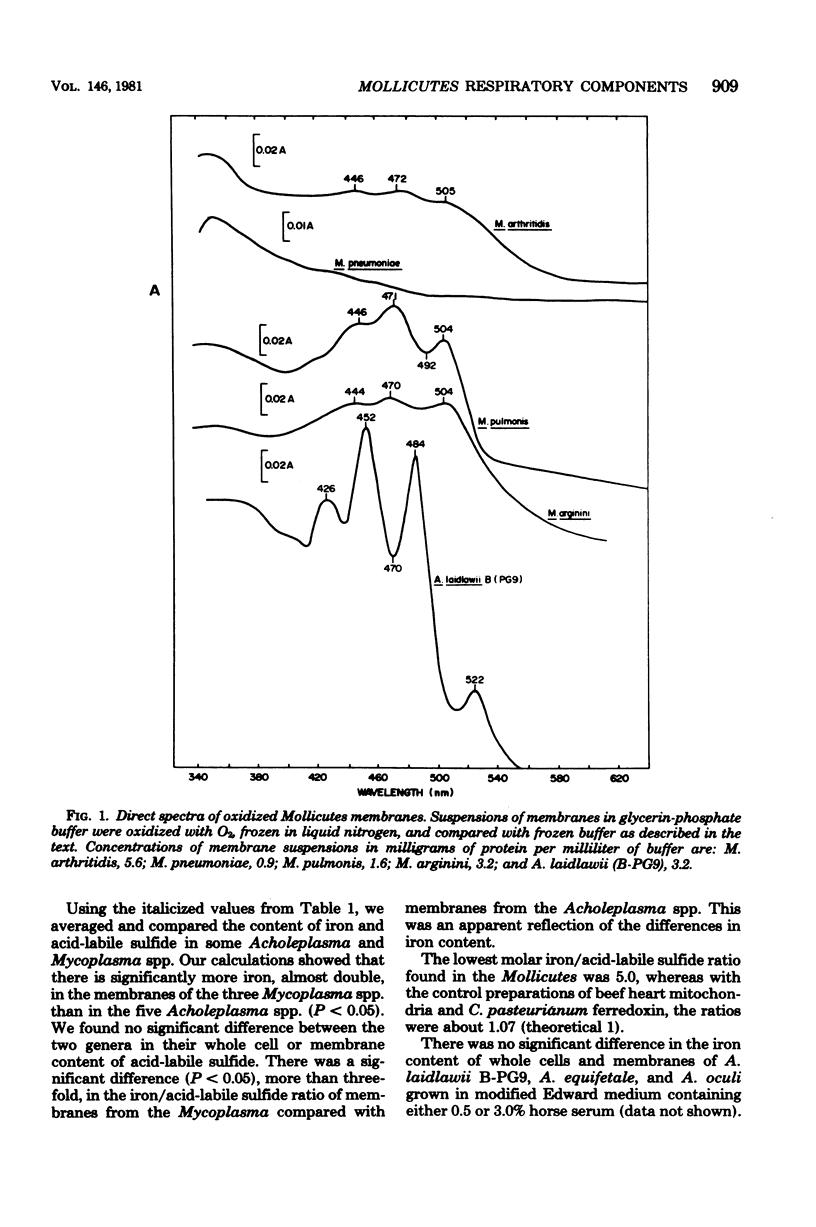
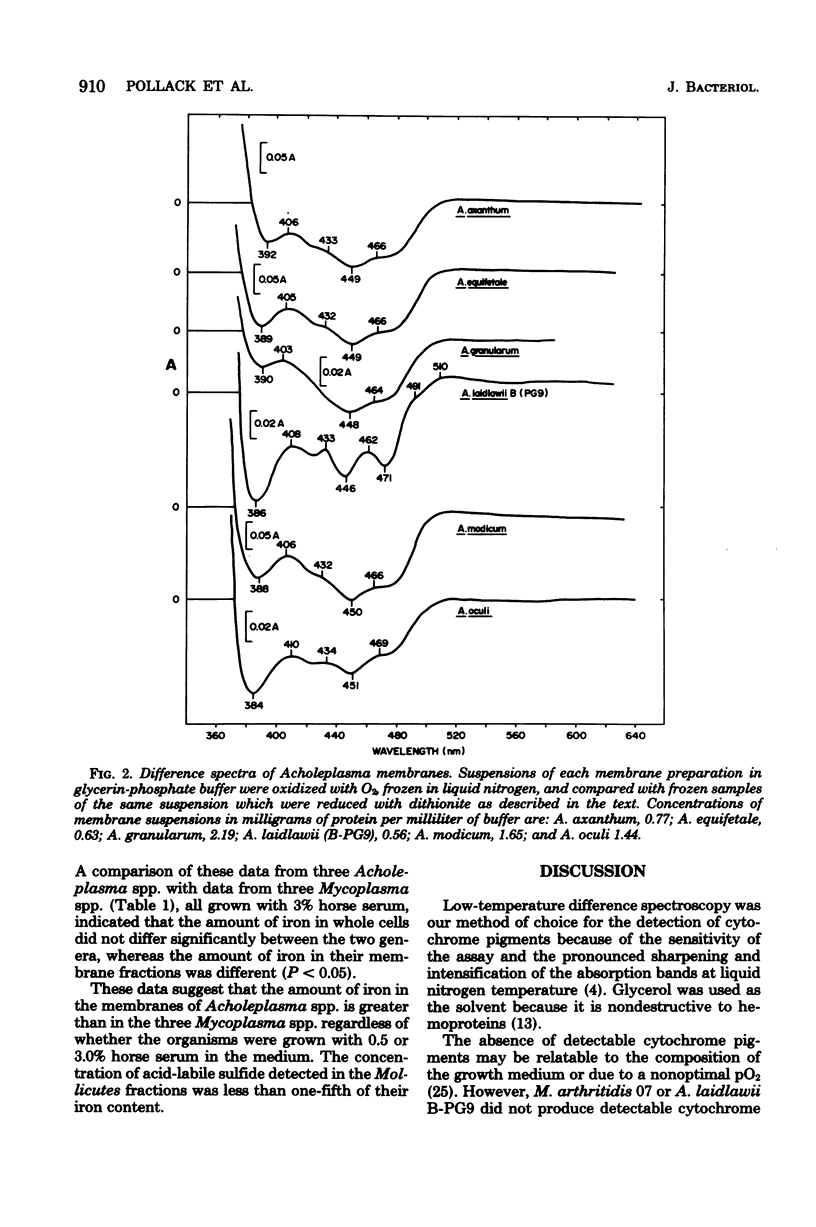
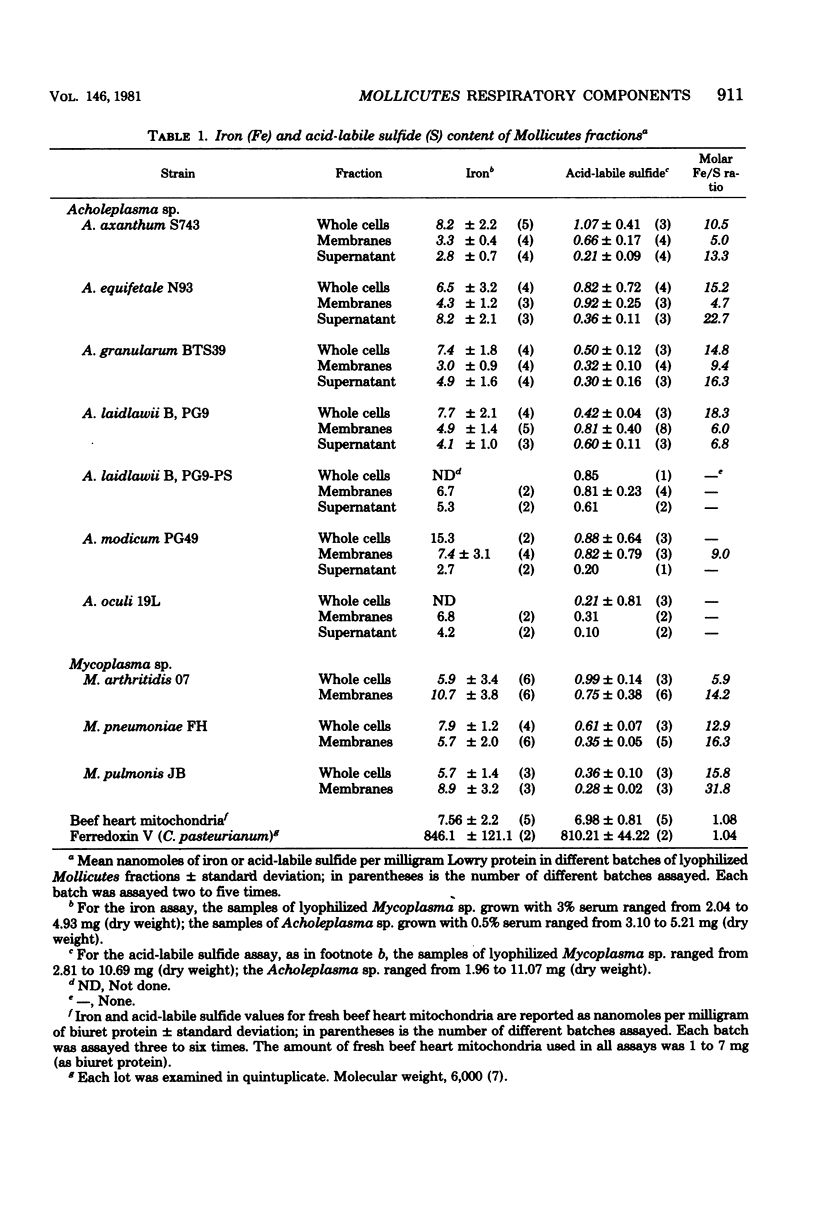


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauminger E. R., Cohen S. G., Labenski de Kanter F., Levy A., Ofer S., Kessel M., Rottem S. Iron storage in Mycoplasma capricolum. J Bacteriol. 1980 Jan;141(1):378–381. doi: 10.1128/jb.141.1.378-381.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTABROOK R. W. The low temperature spectra of hemoproteins. I. Apparatus and its application to a study of cytochrome c. J Biol Chem. 1956 Dec;223(2):781–794. [PubMed] [Google Scholar]
- GREEN D. E., WHARTON D. C. STOICHIOMETRY OF THE FIXED OXIDATION-REDUCTION COMPONENTS OF THE ELECTRON TRANSFER CHAIN OF BEEF HEART MITOCHONDRIA. Biochem Z. 1963;338:335–348. [PubMed] [Google Scholar]
- Hall D. O., Rao K. K., Cammack R. The iron-sulphur proteins: structure, function and evolution of a ubiquitous group of proteins. Sci Prog. 1975 Summer;62(246):285–317. [PubMed] [Google Scholar]
- Hanstein W. G., Hatefi Y. Lipid oxidation in biological membranes. II. Kinetics and mechanism of lipid oxidation in submitochondrial particles. Arch Biochem Biophys. 1970 May;138(1):87–95. doi: 10.1016/0003-9861(70)90287-0. [DOI] [PubMed] [Google Scholar]
- Holländer R., Wolf G., Mannheim W. Lipoquinones of some bacteria and mycoplasmas, with considerations on their functional significance. Antonie Van Leeuwenhoek. 1977;43(2):177–185. doi: 10.1007/BF00395672. [DOI] [PubMed] [Google Scholar]
- Jinks D. C., Matz L. L. The reduced nicotinamide adenine dinucleotide "oxidase" of Acholeplasma laidlawii membranes. Biochim Biophys Acta. 1976 Apr 9;430(1):71–82. doi: 10.1016/0005-2728(76)90223-1. [DOI] [PubMed] [Google Scholar]
- Jung D. W., Chávez E., Brierley G. P. Energy-dependent exchange of K+ in heart mitochondria. K+ influx. Arch Biochem Biophys. 1977 Oct;183(2):452–459. doi: 10.1016/0003-9861(77)90380-0. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Orme-Johnson W. H., Holm R. H. Identification of iron--sulfur clusters in proteins. Methods Enzymol. 1978;53:268–274. doi: 10.1016/s0076-6879(78)53032-2. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Orme-Johnson N. R. Overview of iron--sulfur proteins. Methods Enzymol. 1978;53:259–268. doi: 10.1016/s0076-6879(78)53031-0. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Somerson N. L., Senterfit L. B. Effect of pH on the immunogenicity of Mycoplasma pneumoniae. J Bacteriol. 1969 Feb;97(2):612–619. doi: 10.1128/jb.97.2.612-619.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. D., Somerson N. L., Senterfit L. B. Isolation, Characterization, and Immunogenicity of Mycoplasma pneumoniae Membranes. Infect Immun. 1970 Sep;2(3):326–339. doi: 10.1128/iai.2.3.326-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- Ritchey T. W., Seely H. W., Jr Distribution of cytochrome-like respiration in streptococci. J Gen Microbiol. 1976 Apr;93(2):195–203. doi: 10.1099/00221287-93-2-195. [DOI] [PubMed] [Google Scholar]
- SMITH S. L., VANDEMARK P. J., FABRICANT J. RESPIRATORY PATHWAYS IN THE MYCOPLASMA. I. LACTATE OXIDATION BY MYCOPLASMA GALLISEPTICUM. J Bacteriol. 1963 Nov;86:893–897. doi: 10.1128/jb.86.5.893-897.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. P., Gutman M. The DPNH dehydrogenase of the mitochondrial respiratory chain. Adv Enzymol Relat Areas Mol Biol. 1971;34:79–153. doi: 10.1002/9780470122792.ch3. [DOI] [PubMed] [Google Scholar]
- Smith L. Bacterial cytochromes and their spectral characterization. Methods Enzymol. 1978;53:202–212. doi: 10.1016/s0076-6879(78)53025-5. [DOI] [PubMed] [Google Scholar]
- Somerson N. L., James W. D., Walls B. E., Chanock R. M. Growth of Mycoplasma pneumoniae on a glass surface. Ann N Y Acad Sci. 1967 Jul 28;143(1):384–389. doi: 10.1111/j.1749-6632.1967.tb27680.x. [DOI] [PubMed] [Google Scholar]
- TOURTELLOTTE M. E., JACOBS R. E. Physiological and serologic comparisons of PPLO from various sources. Ann N Y Acad Sci. 1960 Jan 15;79:521–530. doi: 10.1111/j.1749-6632.1960.tb42718.x. [DOI] [PubMed] [Google Scholar]
- Tarshis M. A., Bekkouzjin A. G., Ladygina V. G. On the possible role of respiratory activity of Acholeplasma laidlawii cells in sugar transport. Arch Microbiol. 1976 Sep 1;109(3):295–299. doi: 10.1007/BF00446641. [DOI] [PubMed] [Google Scholar]
- VANDEMARK P. J., SMITH P. F. RESPIRATORY PATHWAYS IN THE MYCOPLASMA. II. PATHWAY OF ELECTRON TRANSPORT DURING OXIDATION OF REDUCED NICOTINAMIDE ADENINE DINUCLEOTIDE BY MYCOPLASMA HOMINIS. J Bacteriol. 1964 Jul;88:122–129. doi: 10.1128/jb.88.1.122-129.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Carithers R. P. Bacterial iron-sulfur proteins. Microbiol Rev. 1979 Sep;43(3):384–421. doi: 10.1128/mr.43.3.384-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


