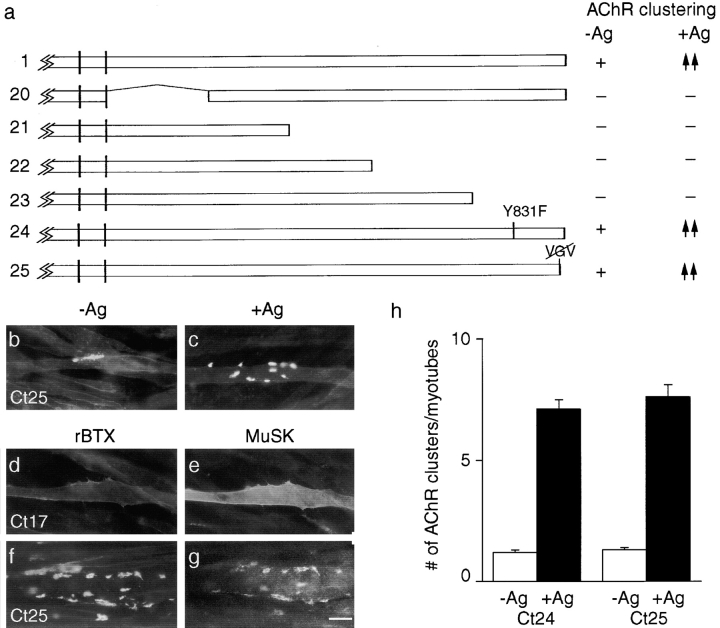
Figure 8.
Binding sites predicted to recognize p85 and PDZ domain-containing proteins are inessential for MuSK to cluster AChRs or to associate with AChR clusters. (a) Mutant constructs. To the right of each construct is indicated whether or not it rescued the ability of MuSK −/−myotubes to form AChR clusters in the absence of agrin (−Ag) or to form additional clusters in the presence of agrin (+Ag). +, spontaneous clustering; −, no clustering; ↑↑, wild-type level of agrin sensitivity; and ↑, reduced clustering relative to wild-type. In constructs 20–23, large segments of the cytoplasmic domain were deleted. In constructs 24 and 25, putative binding sites for p85 and PDZ domains were mutated. Constructs 20–23 were completely inactive, whereas constructs 24 and 25 showed activity equivalent to that of wild-type MuSK. (b and c) Cells that had been transfected with construct 25, incubated with or without agrin, and then stained with rBTX. (d–g) Cells that had been transfected with construct 17 (d and e) or 25 (f and g), treated with agrin, and then doubly stained with rBTX (d and f) and anti-MuSK antibodies (e and g). The MuSK mutant with a three residue carboxy-terminal truncation (construct 25) is unable to bind PDZ domains in PICK (data not shown) but can induce and associate with AChR clusters. MuSK and AChRs are both diffusely distributed in myotubes transfected with construct 17. (h) Quantitation of the extent to which MuSK constructs 24 and 25 rescued the ability of MuSK−/− myotubes to form AChR clusters in the absence or presence of agrin. Cultures were doubly stained with rBTX and anti-MuSK, and only MuSK-positive (i.e., successfully transfected) myotubes were scored. Bar, 20 μm.