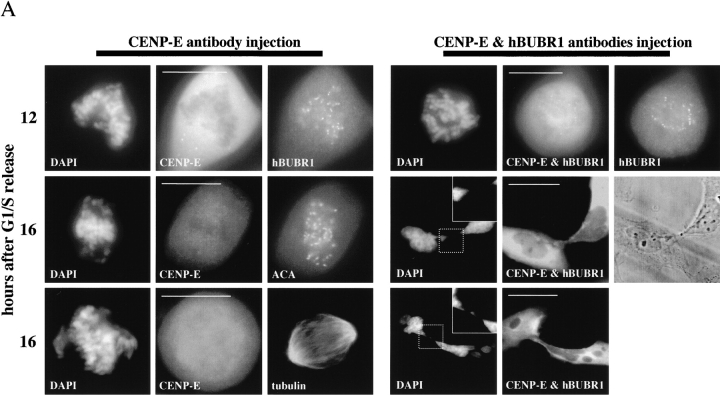
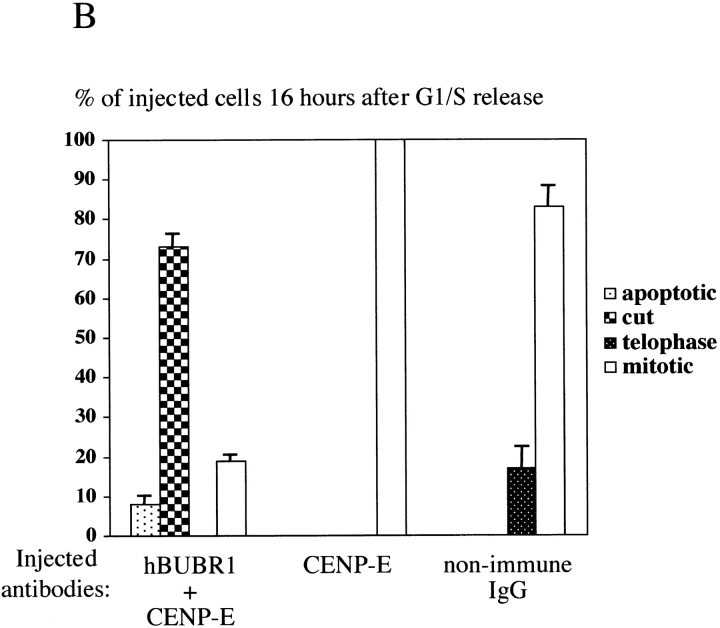
Figure 4.
hBUBR1 is required for mitotic arrest resulting from loss of CENP-E functions at kinetochores. Synchronized Hela cells were injected with rabbit anti–CENP-E and hBUBR1 antibodies and sampled at 12 or 16 h after release from the G1/S boundary. (A) Immunofluorescence staining of injected cells at these time points. (left gallery) Cells injected with CENP-E antibodies arrest in mitosis with unaligned chromosomes. Cells were stained for the injected CENP-E antibodies (middle), localization of endogenous hBUBR1, ACA, and tubulin (rows 1–3, respectively, right). (right gallery) Cells coinjected with CENP-E and hBUBR1 antibodies (middle) enter mitosis with hBUBR1 at kinetochores (right, row 1). At the 16-h time points (rows 2 and 3), the cells coinjected with CENP-E and hBUBR1 antibodies exit mitosis with unaligned chromosomes to produce divided cells with lagging chromosomes (inset in left) and multiple nuclei that reform around unsegregated or incompletely separated chromosomes. Phase-contrast image show cells were tethered at the stembody (row 2, right). Injected antibodies were stained with Cy5 anti-rabbit secondary antibodies (middle columns of both galleries). Endogenous hBUBR1 was stained with anti-rat hBUBR1 and visualized with Cy2 anti-rat secondary antibody. ACA was visualized with Cy2 anti-human secondary antibodies. Microtubules were detected with antitubulin mAbs and Cy2 anti-mouse secondary antibodies. Chromosomes and nuclei were stained with DAPI (left column). (B) Comparison of the fates of cells injected with CENP-E antibodies, hBUBR1+CENP-E antibodies, and nonimmune IgG. After cells were released from the G1/S boundary for 16 h, the percentage of cells injected with the various antibodies that were in mitosis (open bars), normal telophase (white dotted bar), cut (checkered bar), and apoptosis (black dotted bar) were quantitated and compared. The plotted values are the means plus SDs from at least three experiments. Bars, 10 μm.