Figure 2.
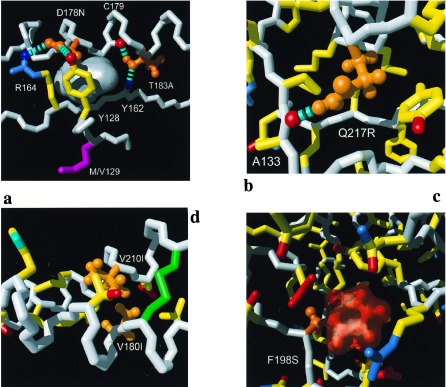
Close-ups of individual mutation sites: (a) D178N and T183A, (b) Q217R, (c) F198S, and (d) V180I and V210I. Color code: white, polypeptide backbone; orange, side chains for which a mutation has been associated with inherited human prion diseases; yellow, side chain carbon—carbon bonds; blue, bonds to side-chain nitrogen atoms and selected backbone amide groups; red, bonds to side-chain oxygen atoms and selected backbone carbonyl oxygens. The hydrogen bonds are represented by broken cyan cylinders. (a) An empty cavity observed in wild-type mPrP(121–231) is shown as a gray surface. (b) All bonds within a slice of approximately 18 Å thickness and (c) those within an 10 Å slice. (c) The orange transparent surface represents the empty space that would be left after the amino acid replacement F198S in the absence of any subsequent structural rearrangement. (d) All side chains of helices 2 and 3 are shown for which the inter-helix distance between at least one pair of side-chain heavy atoms is shorter than 5 Å.
