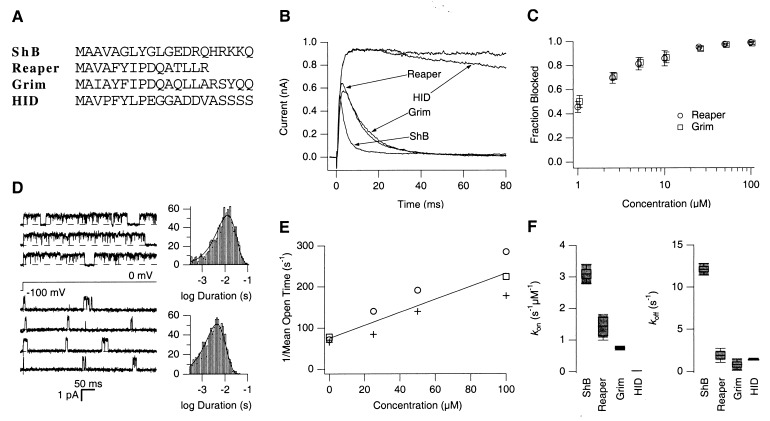
Figure 1.
Short synthetic apoptosis peptides induce inactivation in voltage-dependent ShBΔ6–46:T449V K+ channels. (A) N-terminal sequences of the ShB K+ channel, Reaper, Grim, and Hid. The number of amino acids shown corresponds to the length of synthetic peptides used in the experiments. Peptides were synthesized as described in Materials and Methods. (B) Representative macroscopic currents recorded from an inside-out patch at +50 mV in the presence of the ShB, Reaper, Grim, or Hid peptide. Peptides were applied to the cytoplasmic side of the membrane patch at 100 μM concentration. (C) Concentration dependence of the fractional steady-state block of the ShBΔ6–46:T449V current by the Reaper and Grim N-terminal peptides (mean ± SD, n = 5). Steady-state currents were measured in response to pulses to +50 mV for 200 ms to 1.5 s in duration. Fraction of the steady-state current blocked is plotted as a function of the peptide concentration. (D) Effect of the Reaper N terminus peptide on openings of a single ShBΔ6–46:T449V channel. Representative openings at 0 mV with and without the Reaper peptide are shown. The upper three sweeps were recorded in the absence of and the lower four sweeps were recorded in the presence of Reaper peptide (100 μM). The open time histograms generated from the openings without peptide (upper) and with peptide (lower) also are shown. Solid lines in the histograms represent single exponential fits to the data. The mean open times for the control and peptide data were 12.8 ms and 4.4 ms, respectively. (E) Concentration dependence of the reciprocal of the mean open time. The reciprocals of the mean open times, measured in the presence of different concentrations of the Reaper N-terminal peptide, are plotted as a function of the peptide concentration. The different symbols show the results obtained from three different experiments. The straight line shows the concentration dependence as predicted from the open channel scheme discussed in the text. The slope of the line, which represents kON, was 1.6 s−1 μM−1 and the y intercept was 75 s−1. (F) Rate constants (kON and kOFF) of the block of ShBΔ6–46:T449V currents by the synthetic N-terminal peptides. Rate constants were estimated from macroscopic current recordings as described in Materials and Methods. The estimated values of blocking and unblocking rate constants, kON and kOFF, respectively, are compared by using boxplots.