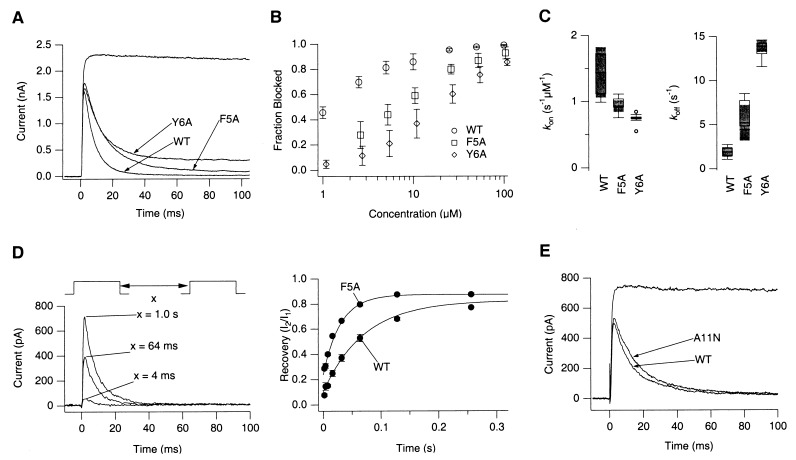
Figure 2.
Mutations known to affect Reaper’s cell killing ability also affect K+ channel blocking ability. (A) Blocking effects of Reaper N terminus peptides (wild-type, F5A, and Y6A) on ShBΔ6–46:T449V currents. Currents recorded from an inside-out patch at +50 mV are shown. Peptides were applied at 100 μM. (B) Concentration dependence of the channel block by the Reaper wild-type, F5A, and Y6A N terminus peptides (mean ± SD, n = 5). Macroscopic ShBΔ6–46:T449V currents were measured in response to depolarization to +50 mV for 200 ms to 1.5 s in duration. Normalized steady-state current amplitudes are plotted as a function of the peptide concentration. (C) Rate constants (kON and kOFF) of the block by the Reaper wild-type, F5A, and Y6A N terminus peptides. Rate constants were estimated by fitting the macroscopic current time course in the presence of the peptides at 100 μM as described in Materials and Methods. Values of the rate constants are compared by using boxplots. (D) Recovery from the Reaper F5A peptide-induced block is faster. Time course of recovery from peptide-induced block was estimated by using a standard double-pulse protocol. Two depolarizing pulses to +50 mV, separated by various intervals as indicated, were applied. Relative current amplitudes, amplitude elicited by the second pulse divided by that from the first pulse, are plotted. Representative ShBΔ6–46:T449V currents recorded in the presence of the Reaper F5A peptide during the second pulses to +50 mV are shown (left). Recovery time courses observed with wild-type and F5A Reaper peptides (100 μM) are compared (right; mean ± SD, n = 3). Smooth lines represent single exponential fits to the data. Recovery time constants for wild-type and F5A Reaper were 70 ms and 30 ms, respectively. (E) The A11N mutation does not affect the Reaper N terminus peptide’s ability to block the channel. Reaper wild-type and A11N peptides were applied at 100 μM. The time courses of the currents recorded in the presence of the two peptides were similar at all concentrations examined (1 to 100 μM, data not shown).