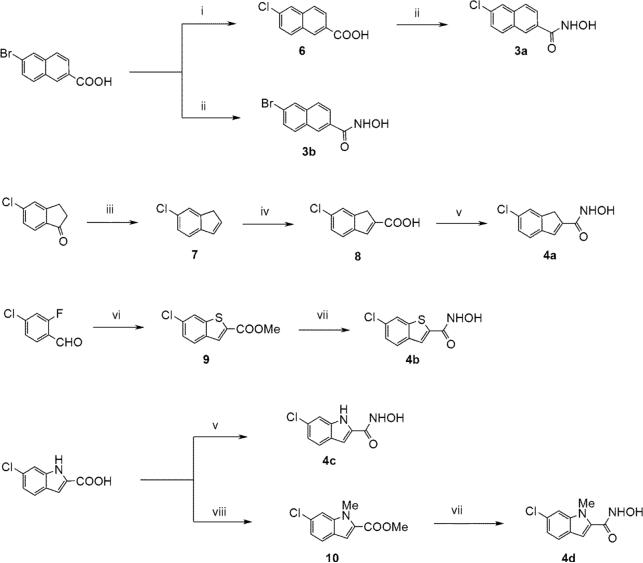
Scheme 1.
Synthesis of ring-fused hydroxamates. Reagents and conditions: (i) CuI, CuCl, DMF, 150 °C, 24h, 63%; (ii) 1. ethyl chloroformate, Et3N, THF, 0 °C to rt, 15min, 2. NH2OH, MeOH, rt, 18h, 2 steps, 47- 60%; (iii) 1. NaBH4, MeOH, 0 °C to rt, 1h, 2. TsOH, toluene, 160 °C, 2h, 2 steps 81%; (iv) oxalyl dibromide, 110 °C, 5h, 11%; (v) 1. (trimethylsilyl)diazomethane, toluene/MeOH, rt, 3h, 2. KCN (cat.), 50% NH2OH (aq.), THF/MeOH, rt, 18h, 2 steps 36−43%; (vi) methyl thioglycolate, NaH, DMSO, rt, 5min, 24%; (vii) KCN (cat.), 50% NH2OH (aq.), THF/MeOH, rt, 18h, 15−37%. (viii) MeI, K2CO3, DMF, 60°C, 24 h, 72%.