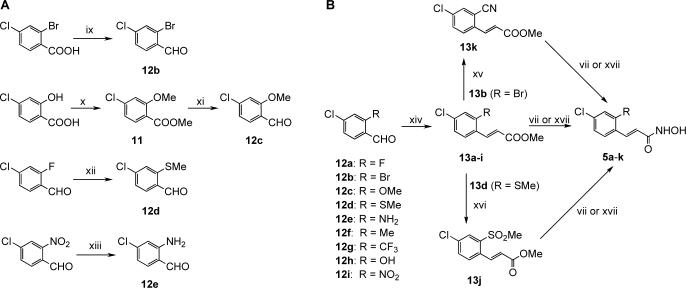
Scheme 2.
Synthesis of ortho-modified hydroxamates. Reagents and conditions: (vii) KCN (cat.), 50% NH2OH (aq.), THF/MeOH, rt, 18h, 15−37%. (ix) 1. BH3-DMS, THF, rt to 70 °C, 3 h, 2. MnO2, CH2Cl2, rt, 18 h, 2 steps 34%; (x) MeI, K2CO3, DMF, 75 °C, 18 h, 95%; (xi) 1. LiBH4, THF, rt to 60 °C, 2 h, 2. MnO2, CH2Cl2, rt, 18 h, 2 steps, 94%; (xii) NaSMe, DMF, rt, 1 h, 91%; (xiii) Fe, HCl, EtOH/AcOH/H2O, 100°C, 15 min., rt, 40 min. (64%); (xiv) methyl diethylphosphonoacetate, NaH, DMF, rt, 18h, 78−99%; (xv) Zn(CN)2, Pd(PPh3)4, DMF, 160°C, 10 min., microwave, (50%); (xvi) Oxone®, MeOH-H2O, 0°C to rt, 5 h, 60%; (xvii) 50% NH2OH (aq.), 1M KOH/MeOH, THF, 0°C, 2−4, 12−58%.