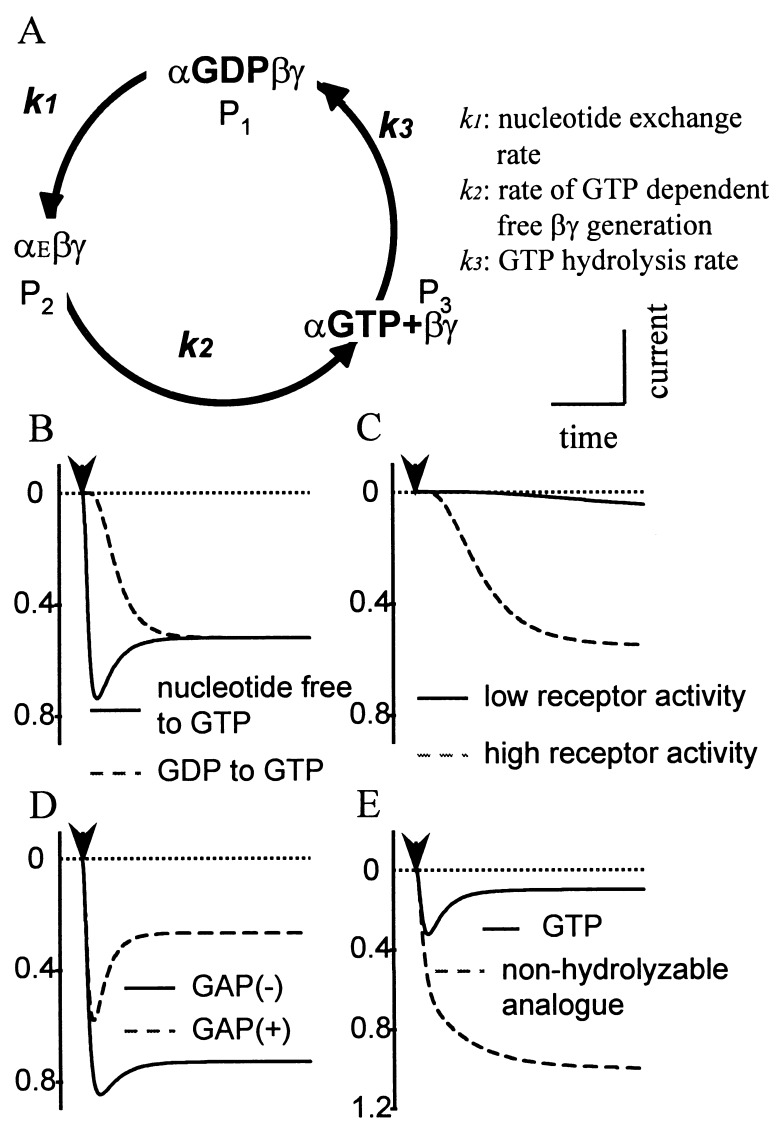
Figure 2.
A hypothetical G-protein turnover cycle is sufficient for the acute desensitization. (A) αEβγ stands for a trimeric G protein with an empty nucleotide binding site. P1, P2, or P3 indicates the fraction of G proteins in each state. The kinetic constants of the G-protein nucleotide exchange-hydrolysis cycle could account for the kinetics of GTP-activated GIRK currents. (B) Slower channel activation is expected when GTP application follows GDP, as compared with following a nucleotide free solution, whereas the steady-state level should be independent of the starting conditions. (C) An increase in the receptor activity speeds up and enhances the activation. (D) The GAP activity alone will contribute to more prominent acute desensitization. (E) Disruption of the G-protein cycle by nonhydrolyzable GTP analogues will lead to greater channel activation and the abolition of acute desensitization.