Figure 7.
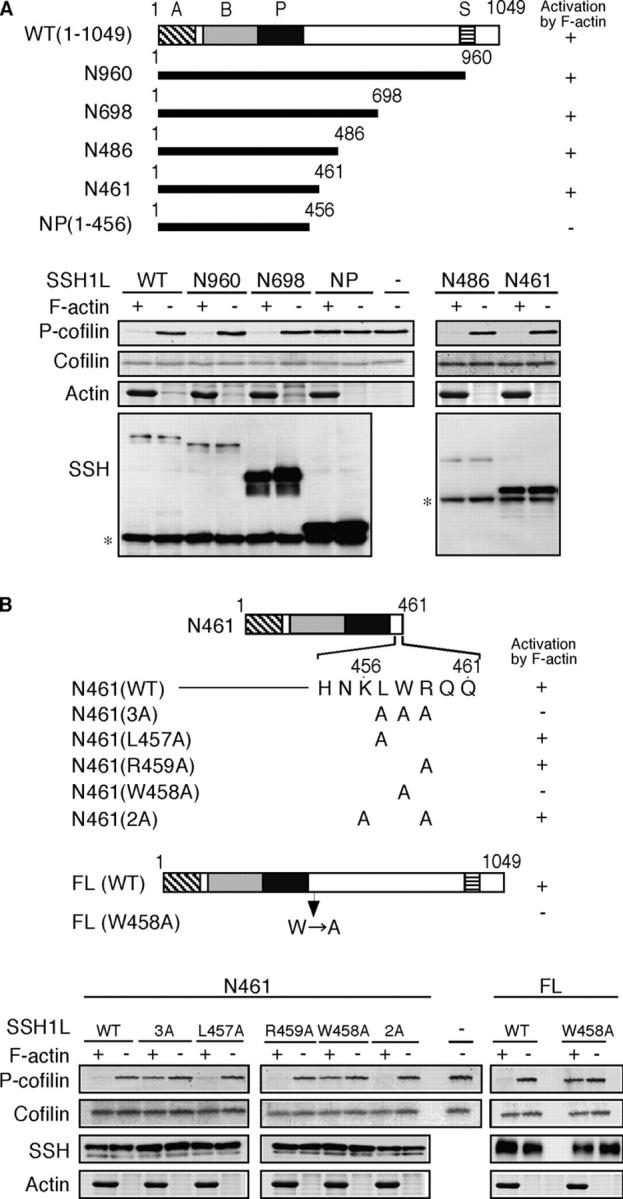
Trp-458 is critical for the F-actin–mediated activation of SSH1L. (A) The role of amino acids 457–461 in F-actin–mediated SSH1L activation. Schematic structures of SSH1L and deletion mutants are shown. The conserved regions in the SSH family are indicated by the A, B, P (phosphatase), and S (Ser-rich) domains. Wild-type (WT) and deletion mutants of (myc + His)-tagged SSH1L were expressed in 293T cells, immunoprecipitated with an anti-myc antibody, and subjected to in vitro phosphatase assays using cofilin-(His)6 as a substrate in the presence or absence of F-actin. P-cofilin levels were measured by Pro-Q staining. Total cofilin and actin were measured by Coomassie blue staining. The expression of SSH1L mutants was analyzed by immunoblotting with the anti-myc antibody. *, Ig heavy chain. (B) Trp-458 is required for F-actin–mediated SSH1L activation. Point mutants of N461 and full-length (FL) SSH1L were subjected to in vitro cofilin phosphatase assays as described in A. Arrow indicates the replacement of Trp-458 with Ala.
